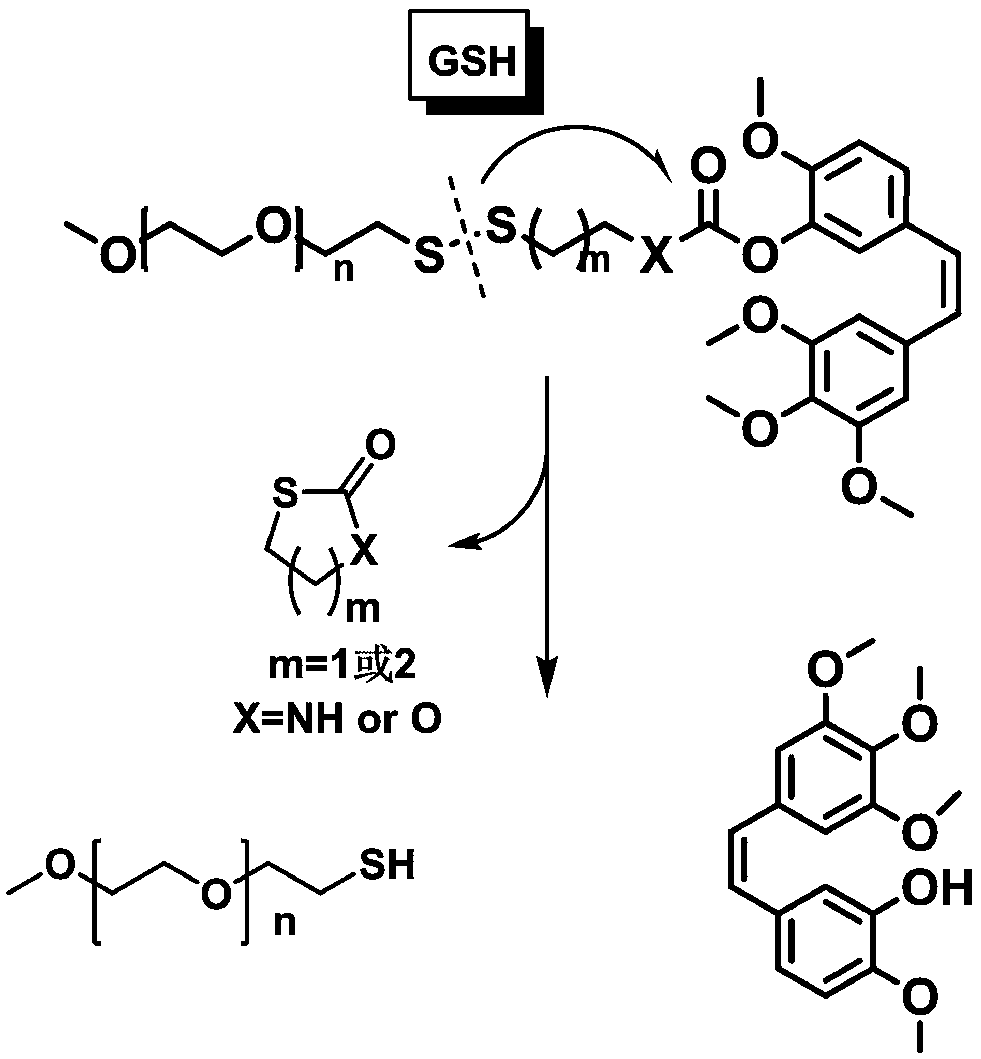
Amphiphilic polymer prodrug of reduced response Combastine and preparation method and application thereof
A technology of amphiphilic polymers and polymers, applied in the direction of drug combinations, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problem of not being able to release the original drug, and achieve mass production, yield and product purity High and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
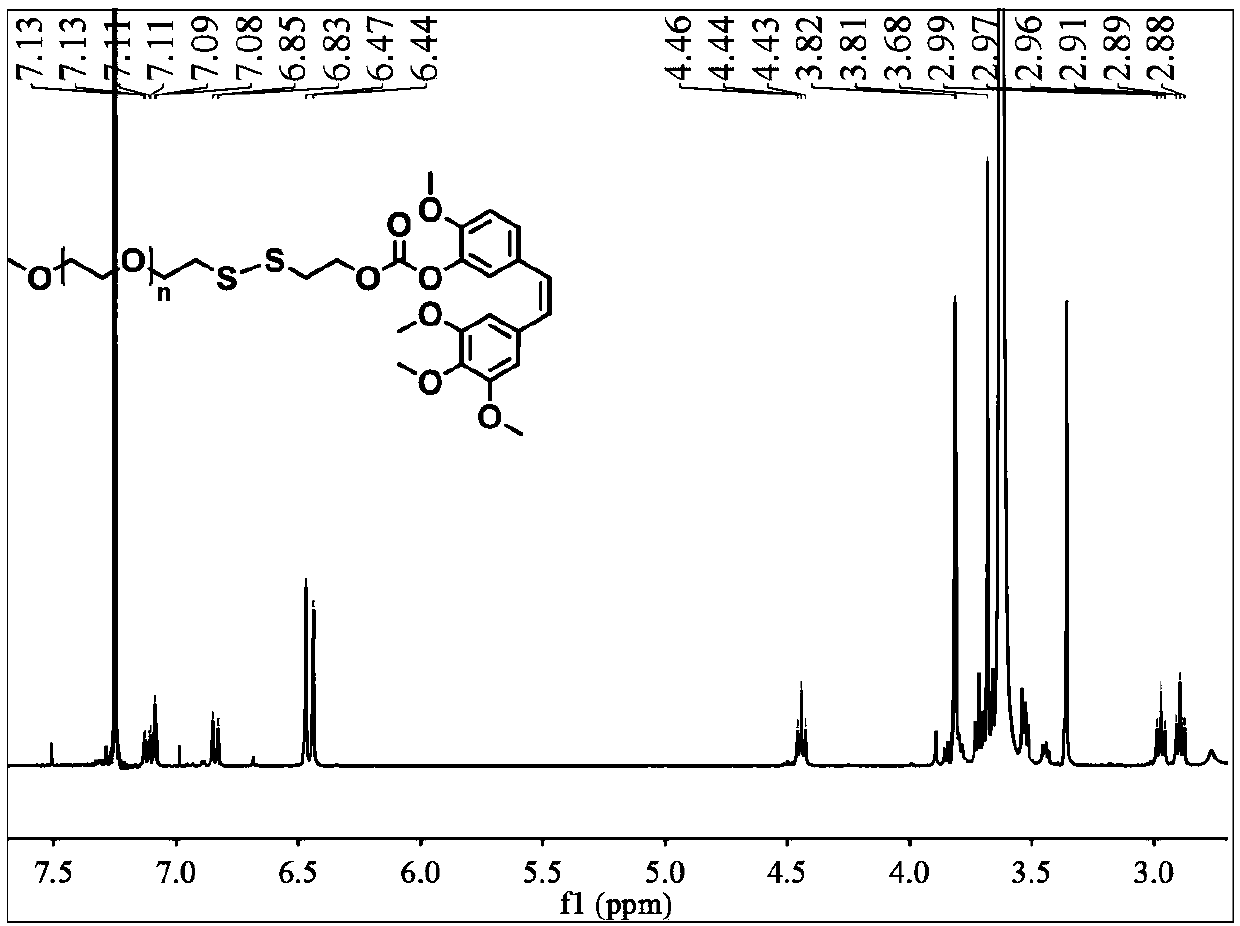
[0039] A reduction-responsive amphiphilic polymer prodrug of combustin has the following molecular structure:
[0040]
[0041] where 5
[0042]The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0043] 1) Preparation of 2-(2-pyridyldithio)ethanol:
[0044] 2-Mercaptoethanol (1.0 g, 12.8 mmol) and 2,2'-dithiobipyridine (Py-SS-Py, 8.46 g, 38.4 mmol) were added into 100 mL of methanol, and stirred at room temperature for 12 hours. Methanol was distilled off under reduced pressure, purified by silica gel column, concentrated and dried in vacuo to obtain 2.2 g of light yellow solid product with a yield of 84%.
[0045] (2) Preparation of 4-nitrophenyl-2-(2-pyridyldithio)ethyl carbonate:
[0046] 2-(2-pyridyldithio)ethanol (2.2g, 10.8mmol) and triethylamine (2.2g, 21.6mmol) were dissolved in 100mL of dichloromethane, and phenyl p-nitrochloroformate ( 6.5g, 32.4mmol) was slowly added dropwise to the solution, after the drop was comple...
Embodiment 2
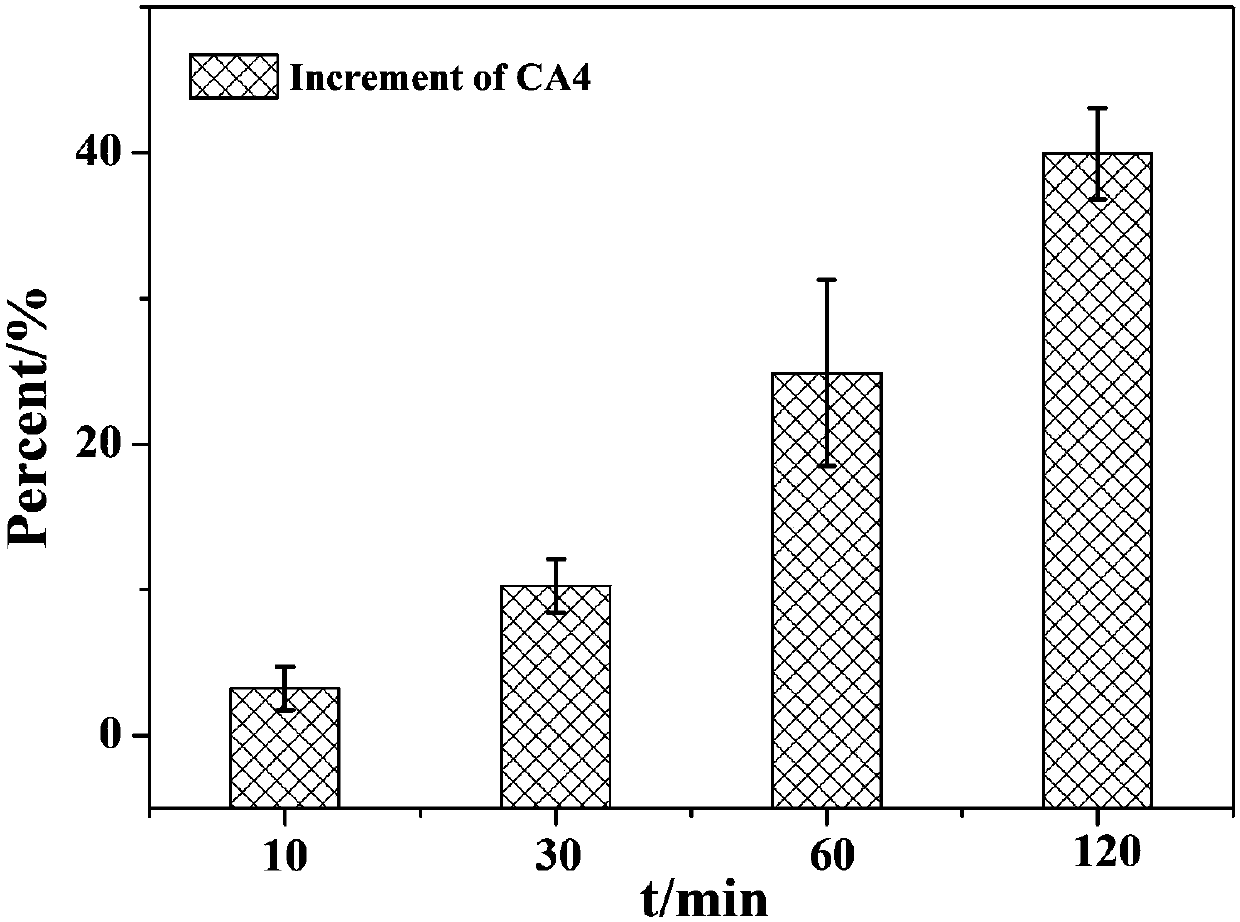
[0055] A nanomicelle containing the amphiphilic polymer prodrug obtained in Example 1 is prepared by the following method: 20 mg of the polymer prodrug and 10 μL of medium-chain fatty acid triglyceride are placed in water, then ultrasonicated for 1 min, and filtered Add mannitol to the membrane and freeze-dry it to obtain the reduction-responsive polymer nanomicelle freeze-dried powder. The particle size and distribution ( Figure 4 ).
Embodiment 3
[0057] A reduction-responsive amphiphilic polymer prodrug of combustin has the following molecular structure:
[0058]
[0059] where 100
[0060] The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0061] 1) Preparation of pyridyl dithioethylamine hydrochloride:
[0062] Add mercaptopropylamine hydrochloride (1.0 g, 7.9 mmol) and 2,2'-dithiobipyridine (Py-SS-Py, 2.3 g, 9.4 mmol) into 60 mL of methanol, and stir at room temperature for 48 hours. The reaction solution was concentrated under reduced pressure, the target product was precipitated with ether, suction filtered, and vacuum-dried to obtain 1.39 g of a light yellow oily liquid product shown in formula e, with a yield of 74.4%.
[0063] 2) Preparation of 4-nitrophenyl-(2-pyridyldithio)aminocarbonate:
[0064] Pyridyldithioethylamine hydrochloride (1.39g, 5.9mmol) and triethylamine (0.72g, 7.1mmol) were dissolved in 50mL of dichloromethane, and phenyl p-nitrochloroformate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com