Reduced response-type amphiphilic polymer prodrug and preparation method and application thereof
A technology of amphiphilic polymers and polymers, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of slow hydrolysis, affecting the anti-tumor effect of drugs, incompleteness, etc., and achieve high yield and product purity , Facilitate mass production, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
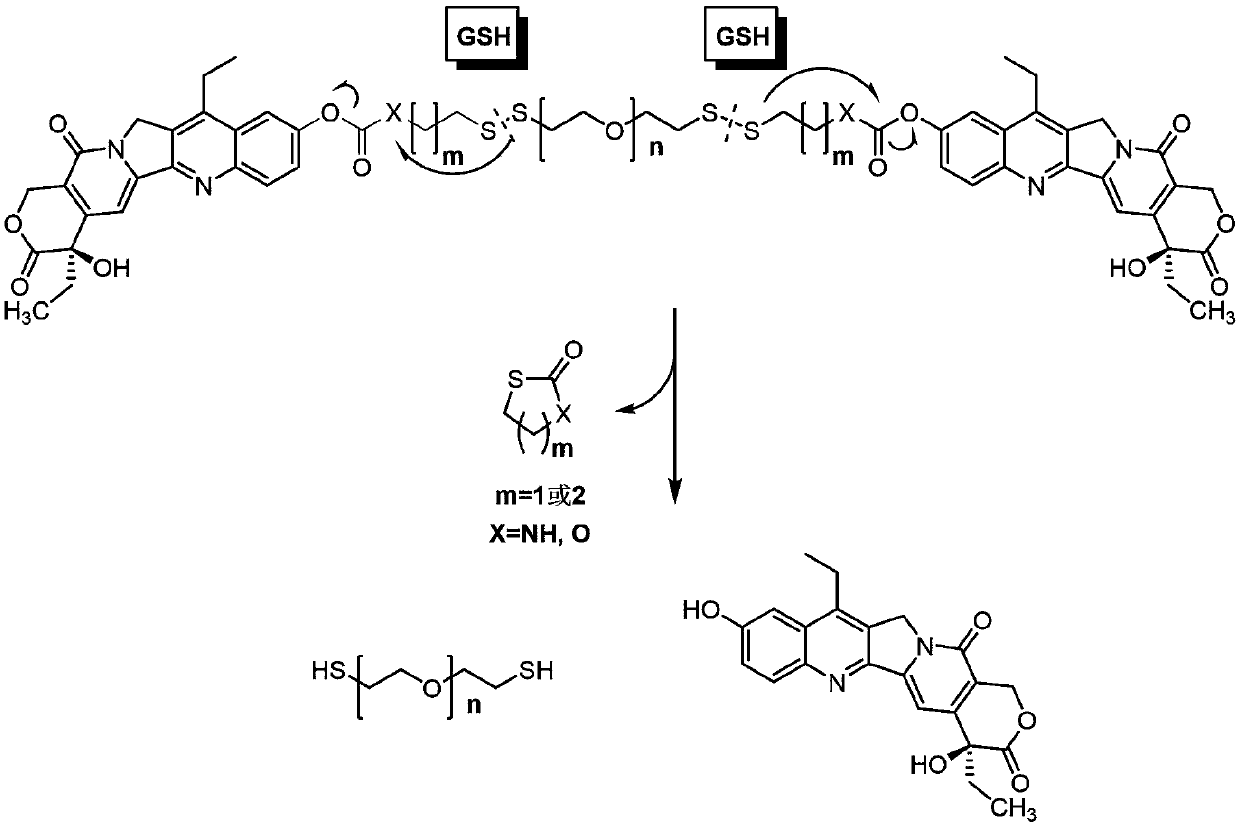
[0043] A reduction-responsive amphiphilic polymer prodrug has the following structural formula:
[0044]
[0045]where 5
[0046] The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0047] 1) Preparation of 2-(2-pyridyldithio)ethanol:
[0048] 2-Mercaptoethanol (0.87g, 11.1mmol) and 2,2'-dithiobipyridine (Py-SS-Py, 2.44g, 11.1mmol) were added into 100mL of methanol, and stirred at room temperature for 12 hours. Methanol was distilled off under reduced pressure, purified by silica gel column, concentrated, and dried in vacuo to obtain 1.6 g of light yellow oily product with a yield of 77.1%. products tested 1 H NMR showed that 2-(2-pyridyldithio)ethanol represented by formula a was obtained.
[0049] 2) Preparation of 4-nitrophenyl-2-(2-pyridyldithio)ethyl carbonate:
[0050] 2-(2-Pyridyldithio)ethanol (1.6g, 8.56mmol) and triethylamine (1.73g, 17.1mmol) were dissolved in 100mL of dichloromethane, and phenyl p-nitrochloroformate...
Embodiment 2
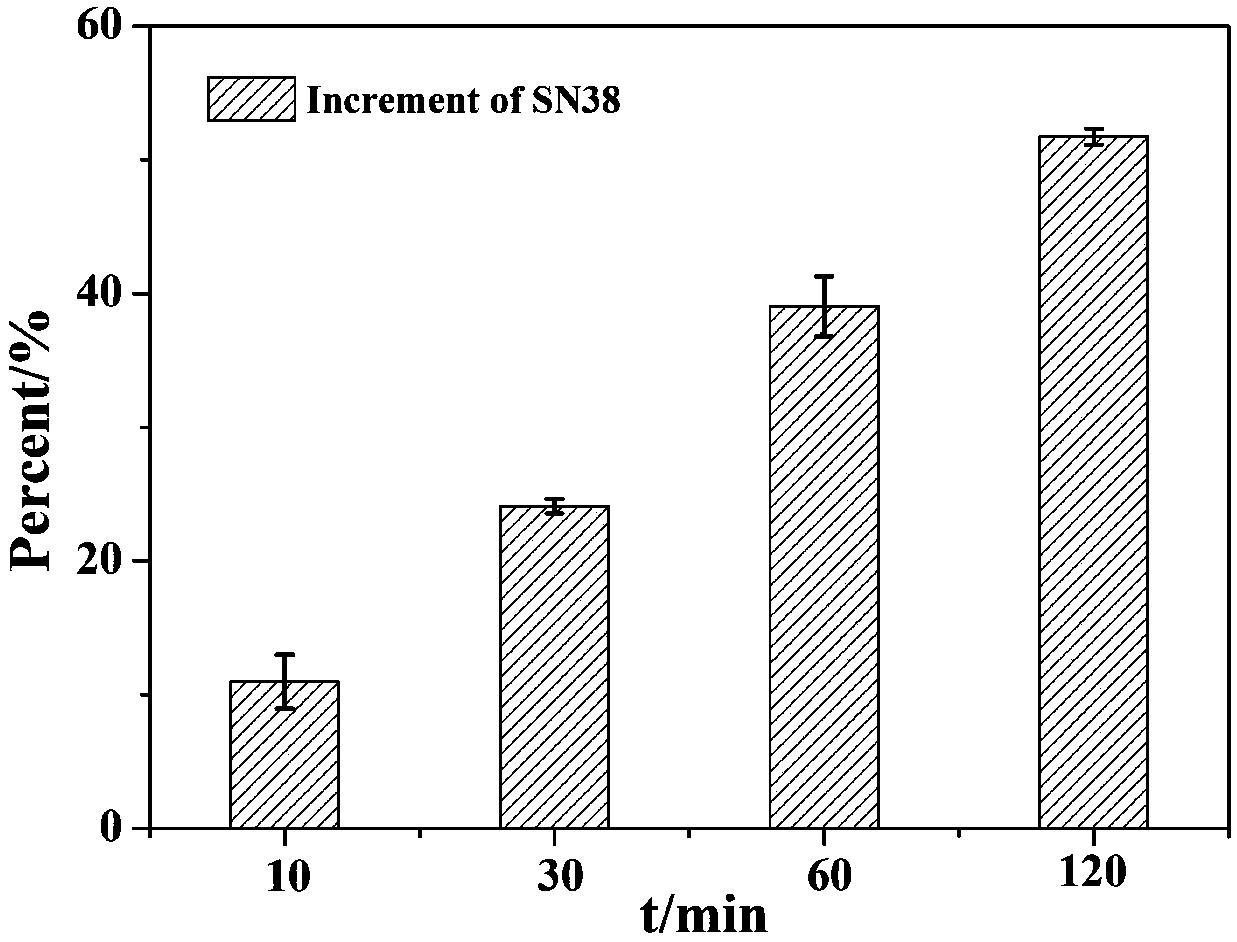
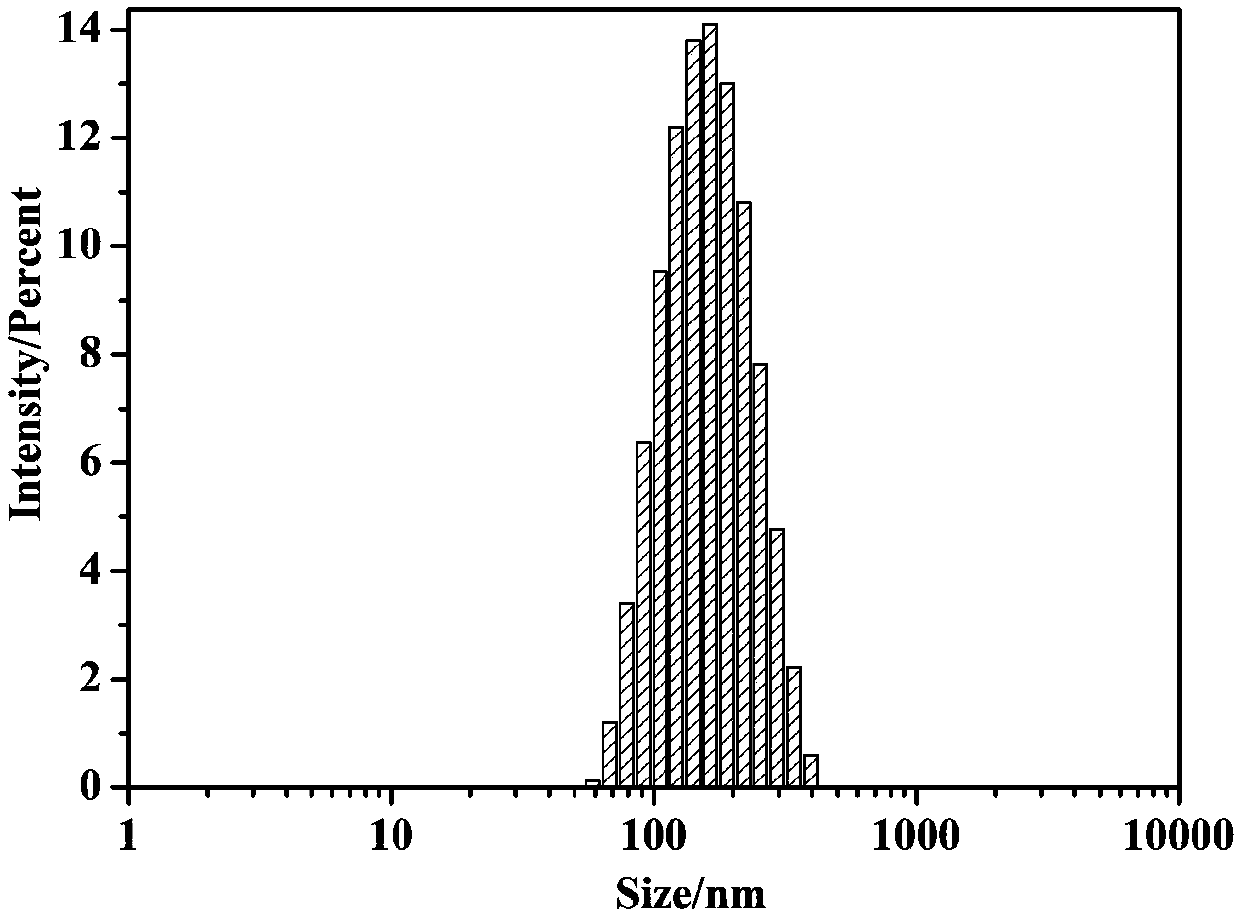
[0059] A nanomicelle containing the amphiphilic polymer prodrug obtained in Example 1 is prepared by the following method: 20 mg of the polymer prodrug and 10 μL of medium-chain fatty acid triglyceride are placed in water, then ultrasonicated for 1 min, and filtered Add mannitol to the membrane and freeze-dry it to obtain the reduction-responsive polymer nanomicelle freeze-dried powder. Use dynamic light scattering (DynamicLight Scattering, DLS) to measure the particle size and distribution of nanomicelle, such as image 3 shown.
Embodiment 3
[0061] A reduction-responsive amphiphilic polymer prodrug has the following structural formula:
[0062]
[0063] where 100
[0064] The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0065] 1) Preparation of 3-(2-pyridyldithio)propanol:
[0066] Add 3-mercaptopropanol (0.5 g, 5.43 mmol) and 2,2′-dithiobipyridine (Py-SS-Py, 2.4 g, 10.9 mmol) into 60 mL of methanol, and stir at room temperature for 24 hours. The methanol was distilled off under reduced pressure, purified by silica gel column, concentrated, and dried in vacuo to obtain 0.87 g of a light yellow oily liquid product shown in formula e, with a yield of 79.7%.
[0067] 2) Preparation of 4-nitrophenyl-3-(2-pyridyldithio)propyl carbonate:
[0068] 3-(2-pyridyldithio)propanol (0.87g, 4.33mmol) and triethylamine (0.52g, 5.15mmol) were dissolved in 50mL of dichloromethane, and p-nitrochloroformic acid benzene was stirred under room temperature The ester (0.87 g, 4.33 mmol)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Drug loading | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com