Amphiphilic polymer prodrug releasing original medicines through reduction response as well as preparation method and applications of amphiphilic polymer prodrug
An amphiphilic polymer and polymer technology, which can be used in drug combinations, pharmaceutical formulations, medical preparations with inactive ingredients, etc. The effect of high yield and product purity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] An amphiphilic polymer prodrug that releases the original drug in response to reduction has the following structural formula:
[0047]
[0048]The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0049] 1) Preparation of 2-(2-pyridyldithio)ethanol:
[0050] 2-Mercaptoethanol (0.87g, 11.1mmol) and 2,2'-dithiobipyridine (Py-SS-Py, 3.63g, 16.5mmol) were added into 100mL of methanol, and reacted at room temperature for 24 hours. Methanol was distilled off under reduced pressure, purified by silica gel column, concentrated and dried in vacuo to obtain 1.6 g of light yellow oily product with a yield of 82.8%. products tested 1 HNMR showed that 2-(2-pyridyldithio)ethanol represented by formula a was obtained.
[0051] 2) Preparation of 4-nitrophenyl-2-(2-pyridyldithio)ethyl carbonate:
[0052] 2-(2-Pyridyldithio)ethanol (1.6g, 9.19mmol) and triethylamine (1.85g, 18.3mmol) were dissolved in 100mL of dichloromethane, and phenyl p-nitrochl...
Embodiment 2
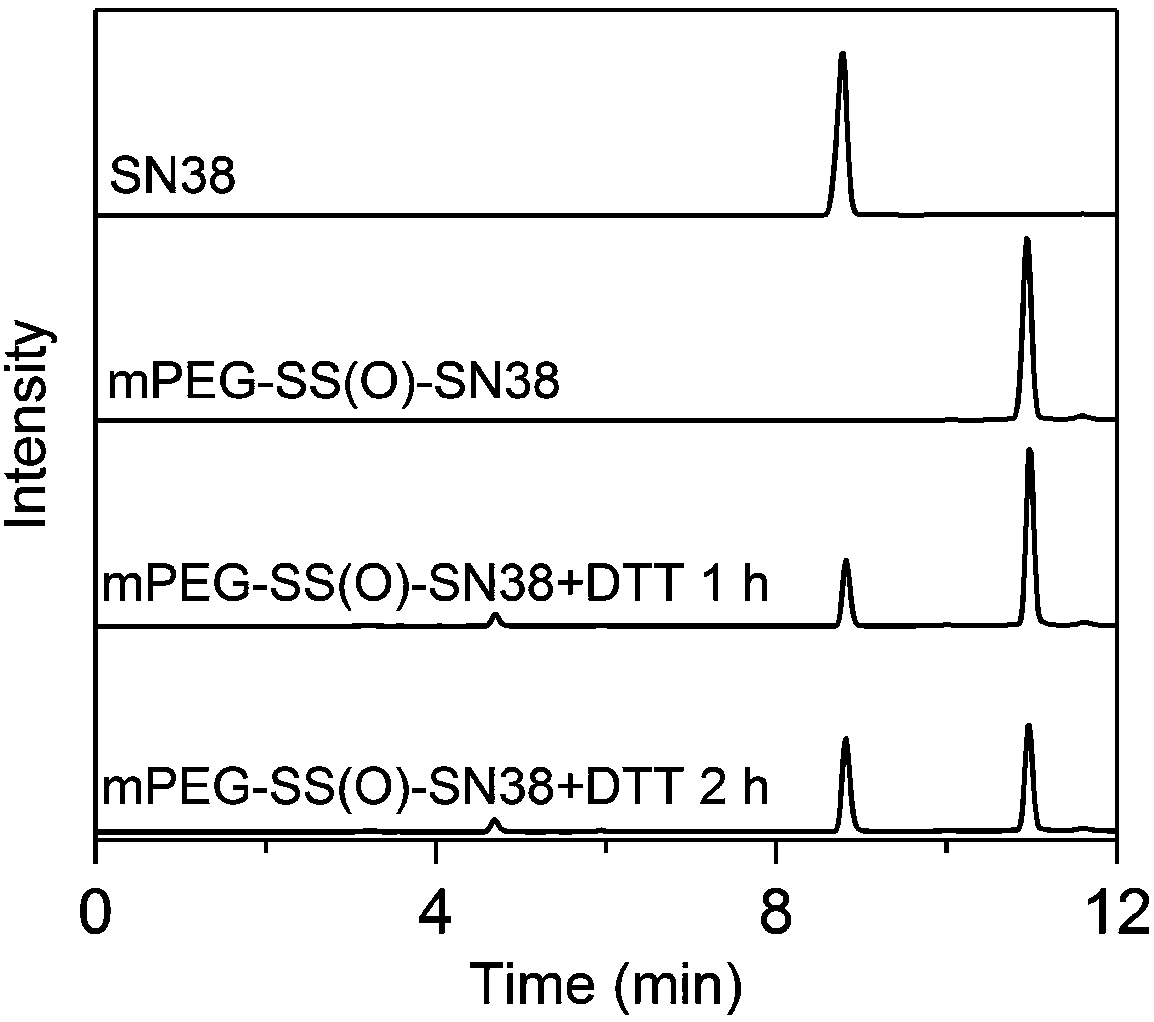
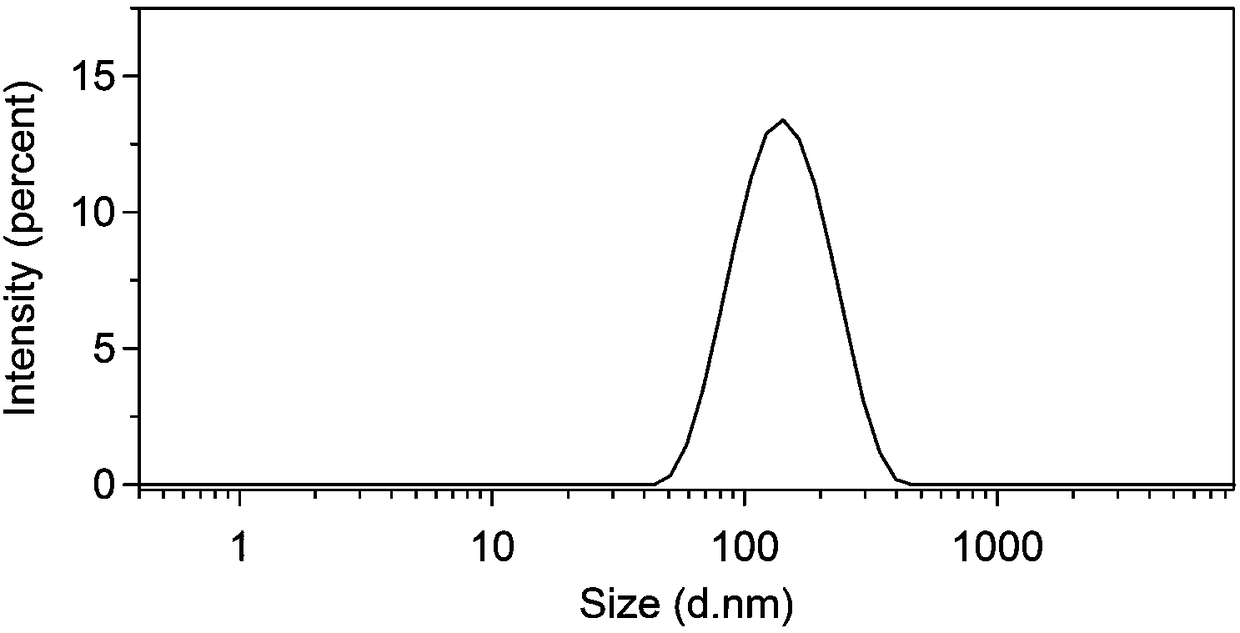
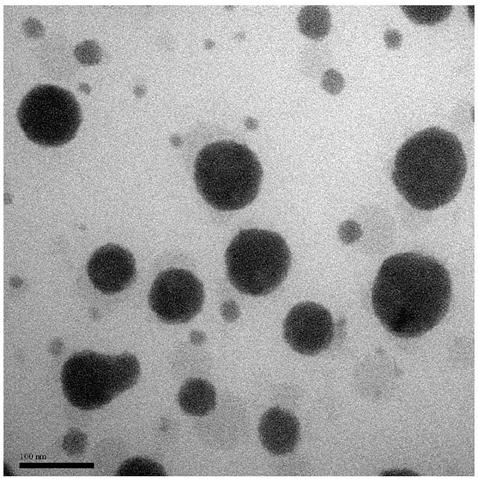
[0060] A nanomicelle containing the amphiphilic polymer prodrug obtained in Example 1 is made by the following method: 1.0 g of the polymer prodrug prepared in Example 1 is dissolved in 50 mL of water, ultrasonicated for 30 seconds, and passed through 0.22 μm filter the membrane, and add 3% (w / v) mannitol to freeze-dry to obtain the reduction-responsive polymer nanomicelle. The particle size and distribution of the nanomicelles were determined by dynamic light scattering (Dynamic Light Scattering, DLS), such as figure 2 shown; and observe the morphology of polymer nanomicelles with a transmission electron microscope (Transmission Electron Microscope, TEM), such as image 3 shown.
Embodiment 3
[0062] An amphiphilic polymer prodrug that releases the original drug in response to reduction has the following structural formula:
[0063]
[0064] The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0065] 1) Preparation of 3-(2-pyridyldithio)propanol:
[0066] 3-Mercaptopropanol (0.5 g, 5.43 mmol) and 2,2′-dithiobipyridine (Py-SS-Py, 2.4 g, 10.8 mmol) were added into 60 mL of methanol and reacted at room temperature for 24 hours. React at room temperature for 24 hours. The methanol was distilled off under reduced pressure, purified by silica gel column, concentrated, and dried in vacuo to obtain 0.87 g of a light yellow oily liquid product shown in formula e, with a yield of 79.7%.
[0067] 2) Preparation of 4-nitrophenyl-3-(2-pyridyldithio)propyl carbonate:
[0068] 3-(2-pyridyldithio)propanol (0.87g, 4.33mmol) and triethylamine (0.52g, 5.15mmol) were dissolved in 50mL of dichloromethane, and p-nitrochloroformic acid benzene was s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com