Posaconazole solid dispersion composition capable of inhibiting separation by crystallization as well as preparation method thereof
A technology of posaconazole solid and saconazole solid, applied in the field of posaconazole solid dispersion composition and its preparation, can solve problems affecting drug absorption effect, drug precipitation, etc., and achieve strong crystallization inhibition ability, Good absorption in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
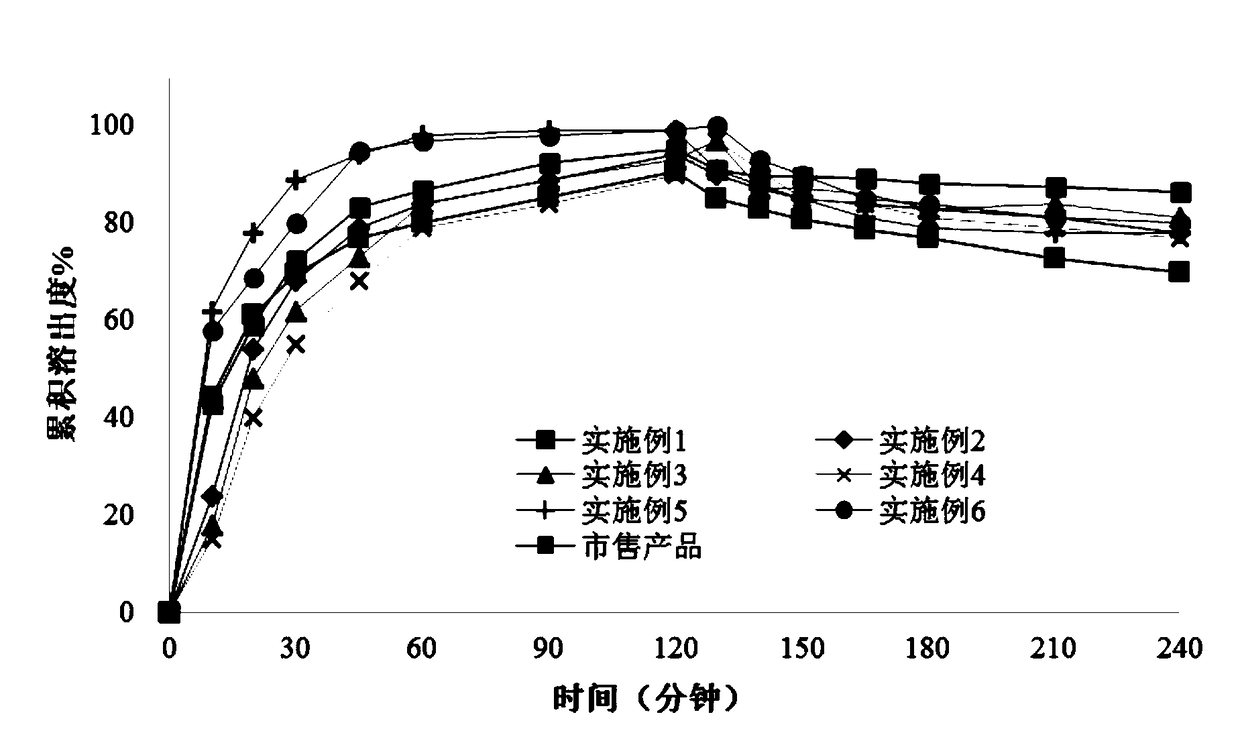
Image
Examples
Embodiment 1
[0047] The posaconazole solid dispersion composition provided in this embodiment contains: posaconazole 100g, methacrylic acid copolymer type C (trade name: Eudragit L 100-55) 325g, hydroxypropyl cellulose ( Molecular weight 80000) 75g, microcrystalline cellulose 70g, croscarmellose sodium 25g, magnesium stearate 5g and Opadry 24g.
[0048] The preparation method of above-mentioned posaconazole solid dispersion composition is as follows:
[0049] Step 1: The active drug posaconazole, the enteric-coated carrier material methacrylic acid copolymer type C and the plasticizer hydroxypropyl cellulose are pre-mixed respectively to form a mixture, wherein the mixing speed is 10 rpm and the mixing time is 20 minutes;
[0050] Step 2: Set the parameters of the hot-melt extruder, set the working temperature of the hot-melt extrusion to 130°C, the temperature of the extrusion port to 140°C, and the screw speed to 150rpm;
[0051] Step 3: Adjust the material delivery rate to achieve a co...
Embodiment 2
[0058] The posaconazole solid dispersion composition provided in this embodiment contains: posaconazole 100g, methacrylic acid copolymer type A (trade name: Eudragit L 100) 325g, hydroxypropyl cellulose (molecular weight 120000 ) 75g, microcrystalline cellulose 70g, croscarmellose sodium 25g, magnesium stearate 5g and Opadry 24g.
[0059] The preparation method of the posaconazole solid dispersion composition provided in this example is the same as that in Example 1.
Embodiment 3
[0061] The posaconazole solid dispersion composition provided in this embodiment contains: posaconazole 100g, methacrylic acid copolymer type C (trade name: Eudragit L 100-55) 325g, hydroxypropyl cellulose ( Molecular weight 100000) 75g, microcrystalline cellulose 70g, croscarmellose sodium 25g, magnesium stearate 5g and Opadry 24g.
[0062] The preparation method of the posaconazole solid dispersion composition provided in this example is the same as that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com