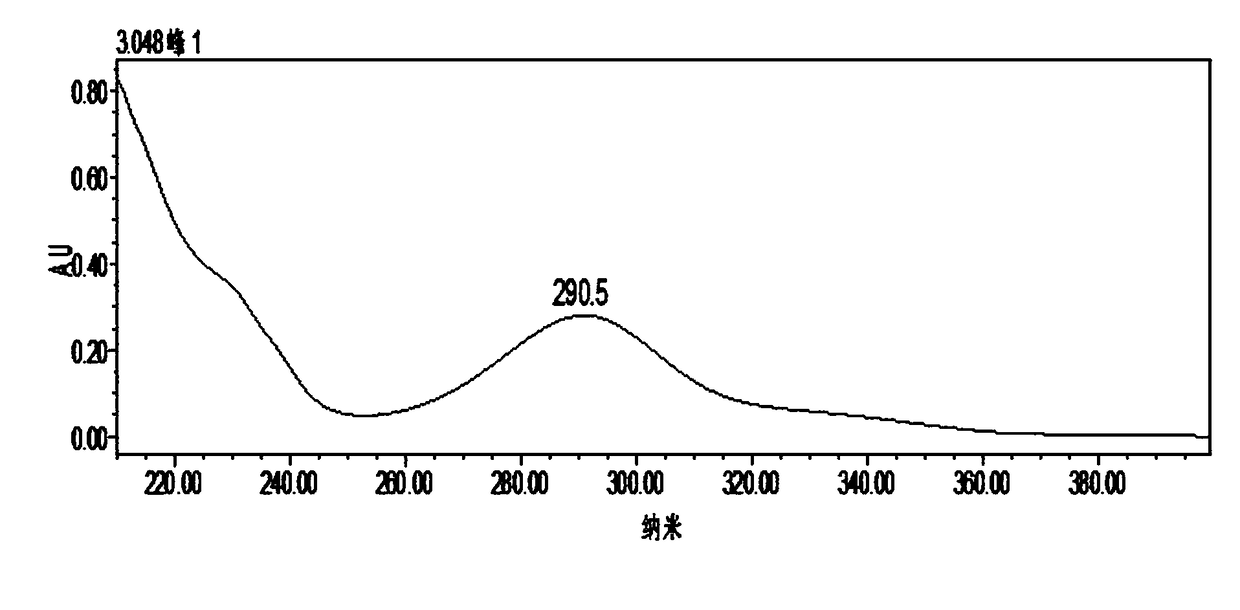
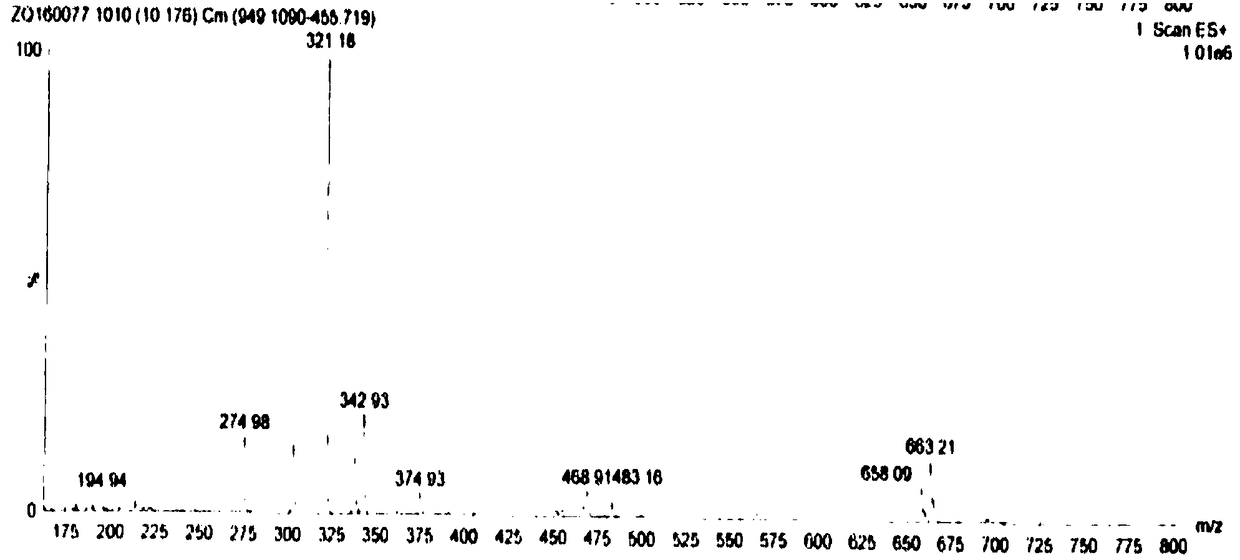
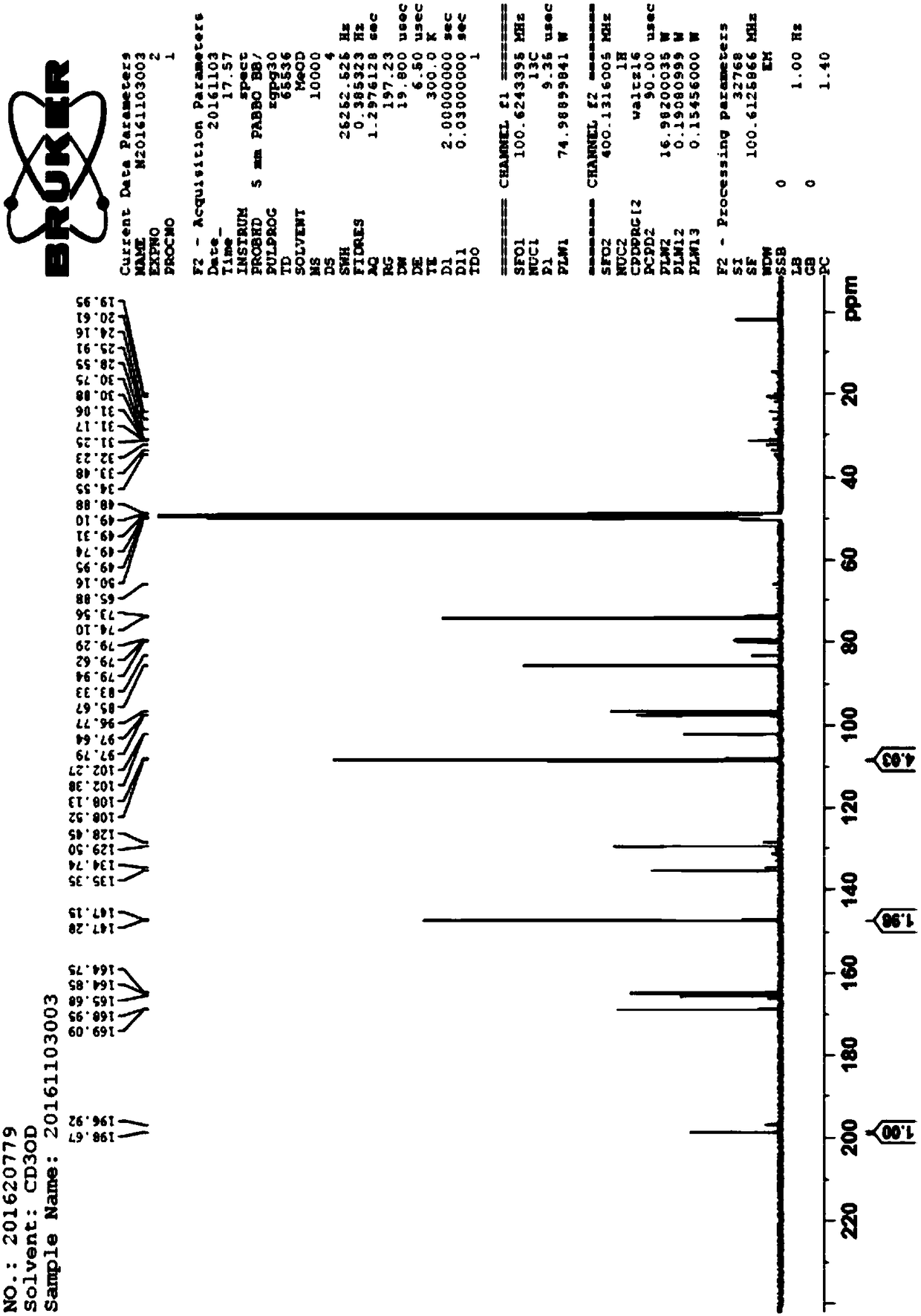
Application of dihydromyricetin in preparation of drug for treating kidney cancer
A kind of technology of dihydromyricetin and therapeutic drug, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, the preparation of dihydromyricetin in Hovenia dulcis fruit
[0026] Dried Hovenia dulcis 1.7kg, crushed through a 100-mesh sieve, added 1.7L of 60% ethanol to reflux extraction at 90°C for 2 hours, filtered with suction, collected extract 1 and filter residue 1; obtained filter residue 1 and 1.7L Mix 60% ethanol, reflux extraction at 90°C for 2 hours, suction filter, recover extract 2 and filter residue 2, mix filter residue 2 with 1.7L 60% ethanol, reflux extraction at 80°C for 2 hours, pump Filtrate and recover to obtain extract 3 and filter residue 3; combine extract 1, extract 2, and extract 3, and concentrate the extract under reduced pressure at 90 ° C to obtain 170.6 g of ethanol crude extract dry powder; take 150 g of Hovenia dulcis fruit crude extract Suspend in 1.5L of distilled water, extract with 4.5L of ethyl acetate, centrifuge the emulsified layer at 5000r / min, 4°C for 10min to separate into aqueous phase 1 and organic phase 1, pour out org...
Embodiment 2
[0028] Embodiment two, the preparation of dihydromyricetin in rattan tea
[0029] Grind 1700g rattan tea, pass through a 100-mesh sieve, add 1.7L 60% ethanol to reflux extraction at 90°C for 2 hours, filter with suction, collect extract 1 and filter residue 1; filter residue 1 obtained with 1.7L 60% Mix ethanol, reflux extraction at 90°C for 2 hours, suction filtration, and recover extract 2 and filter residue 2, mix filter residue 2 with 1.7L 60% ethanol, reflux extraction at 80°C for 2 hours, suction filtration, Recover extract 3 and filter residue 3; combine extract 1, extract 2, extract 3, and concentrate the extract under reduced pressure at 90°C to obtain 153.2 g of ethanol crude extract dry powder; take 150 g of vine tea crude extract and suspend in Extract 1.5L of distilled water with 4.5L of ethyl acetate, centrifuge the emulsified layer at 5000r / min, 4°C for 10min to separate into aqueous phase 1 and organic phase 1, pour out organic phase 1; 4.5L of ethyl acetate w...
Embodiment 3
[0030] Embodiment three, dihydromyricetin and Zn 2+ 、Cu 2+ 、Ni 2+ Reactive combination to form a complex
[0031] Accurately weigh three parts of 60mg (0.2mmol) of the dihydromyricetin composition obtained in Example 1 and add respectively in three 50ml round bottom flasks, add 20ml of absolute ethanol each, stir fully, add sodium acetate to adjust the pH to 7.5, each Add the same amount of zinc acetate, copper acetate, and nickel acetate, heat the flask in a 60°C water bath, condense and reflux for 6 hours, after the reaction is complete, a yellow substance precipitates out, filter, wash with absolute ethanol and water several times, and dry in vacuum . Finally, a yellow insoluble in ethanol, soluble in water complex, dihydromyricetin Zn 2+ Complex 80mg, Dihydromyricetin Cu 2+ Complex 78.6mg, Dihydromyricetin Ni 2+ Complex 75.6 mg.
[0032]The complexes of dihydromyricetin reacted with Zn2+, Cu2+, and Ni2+ are all amorphous yellow powders, which are 312°C, 308°C, and 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com