A kind of recombinant pectin methylesterase pmea and its coding gene and application
A pectin methylesterase and gene technology, applied in the field of genetic engineering, can solve the problems of slow reaction and destruction of pectin polymerization degree and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Recombinant expression of recombinant pectin methylesterase
[0044] 1. Acquisition of recombinant pectin methylesterase gene pmeA:
[0045] Refer to the amino acid sequence of Aspergillus niger pmeA published on GenBank in NCBI. Then, according to the codon preference of Candida utilis, the highest frequency codon was used to optimize the codon of the gene sequence.
[0046] In order to facilitate subsequent molecular cloning, a Bsp119I restriction site was added to the 5' end of the above sequence, and an XbaI restriction site was added to the 3' end. The optimized sequence was synthesized by General Biosystems (Anhui) Co., Ltd.
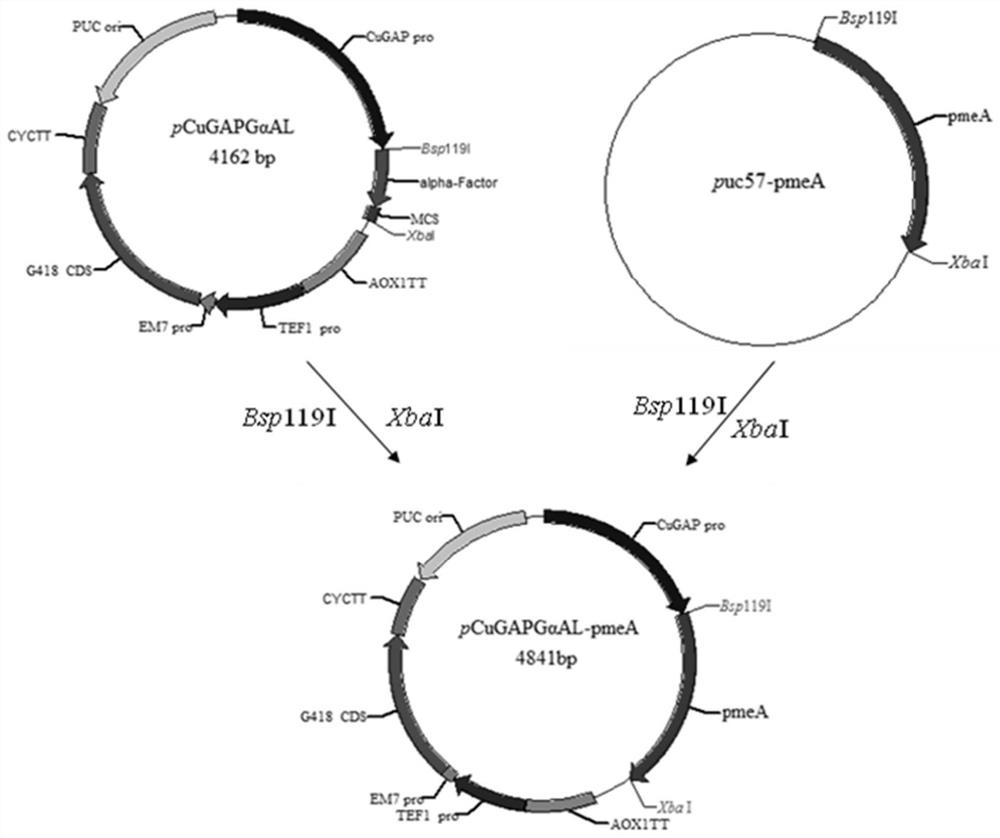
[0047] 2. The pmeA gene fragment was cloned into the pCuGAPGαAL expression vector:
[0048] (1) For the extraction of the pUC57-pmeA plasmid, refer to the operating instructions of the US-based plasmid mini-quick extraction kit.
[0049] (2) Double digestion of plasmids pUC57-pmeA and pCuGAPGαAL.
[0050] Digest pUC57-pmeA ...
Embodiment 2
[0100] Embodiment 2: Enzyme characteristic analysis of recombinant pectin methylesterase
[0101] 1. SDS-PAGE analysis and activity staining of recombinant pectin methylesterase
[0102] Inoculate the positive Candida utilis recombinant bacteria into 5 mL of YPD medium containing G418 with 2% inoculum; after culturing for 24 hours at 30°C and 200 rpm, transfer to 200 mL of YPD medium containing G418 to make the initial OD 600 0.1; after culturing for 96 hours, centrifuge at 8,000×g for 30 minutes to separate the bacteria and supernatant;
[0103] Concentrate the supernatant at 5,000×g at 4°C with a 10k ultrafiltration tube, and concentrate 100 times. Carry out protein quantification, SDS-PAGE electrophoresis and activity staining analysis on the concentrate, such as Figure 4 .
[0104] The principle of active dyeing is that after protein gel refolding, under the optimal conditions of recombinant pectin methylesterase, the recombinant enzyme will react with pectin in protei...
Embodiment 3
[0150] Embodiment 3: the high-density cultivation of recombinant Candida utilis
[0151] 1. Inoculate the recombinant Candida utilis in 5 mL of YPD medium containing G418 at an inoculum size of 2%, culture at 30°C, 200 rpm, for 24 hours;
[0152] 2. After the recombinant bacteria grow to the logarithmic phase, transfer it to 50mL YPD medium containing G418 at 2%, and after cultivating overnight, transfer it to 150mL YPDG medium containing G418 to make the initial OD 600 cultured at 0.1, 30°C, 200rpm;
[0153] 3. When the glycerin content is lower than 0.5%, add 10% glycerin to make the glycerin content 1%;
[0154] 4. After culturing for 168 hours, measure the OD 600 ;
[0155] 5. Collect the bacterial liquid, centrifuge at 5,000×g at 4°C for 30 minutes, collect the supernatant and bacterial cells, and measure the protein concentration in the supernatant and the enzyme activities of endopolygalacturonase and pectin methylesterase respectively.
[0156] The results of enzym...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com