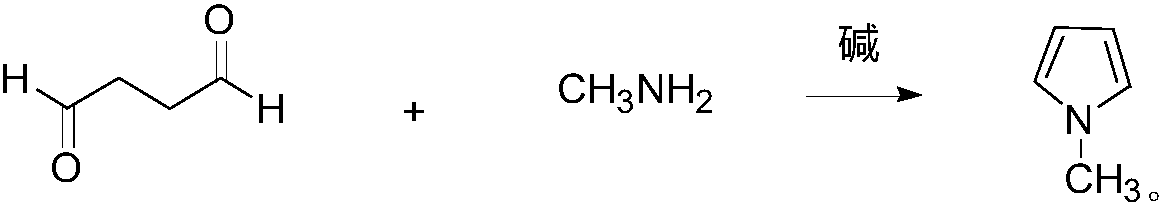
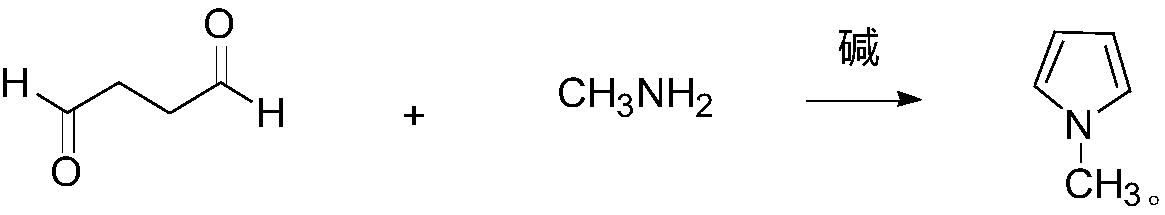Synthesis method of N-methylpyrrole
A technology of methylpyrrole and synthetic method, applied in the direction of organic chemistry, can solve the problems of short specification and route, harsh reaction conditions, low cost, etc., and achieve the effect of short reaction route, convenient post-processing and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of synthetic method of N-methylpyrrole of the present embodiment comprises the following steps: feed N into the reaction bottle 2 , add 3.5g of sodium hydroxide and 54.1g (575.0mmol) of methylamine in ethanol (33wt.%), stir, cool to -10°C, and dropwise add 45g (522.7mmol) of succinic dialdehyde. After the dropwise addition was completed, the temperature was naturally raised to room temperature and then to 60° C., and the stirring reaction was continued for 10 hours. After the reaction was finished, 38.2 g of the product N-methylpyrrole was obtained by atmospheric distillation, with a GC content of 98.6% and a yield of 88.8%.
Embodiment 2
[0028] A kind of synthetic method of N-methylpyrrole of the present embodiment comprises the following steps: feed N into the reaction bottle 2 , adding 3.5g of sodium hydroxide and 73.8g (784.1mmol) of methylamine in ethanol (33wt.%), stirred, cooled to -5°C, and added dropwise 45g (522.7mmol) of succinic dialdehyde. After the dropwise addition was completed, the temperature was naturally raised to room temperature and then to 45° C., and the stirring reaction was continued for 14 hours. After the reaction was finished, 38.6 g of the product N-methylpyrrole was obtained by atmospheric distillation, with a GC content of 98.1% and a yield of 89.3%.
Embodiment 3
[0030] A kind of synthetic method of N-methylpyrrole of the present embodiment comprises the following steps: feed N into the reaction bottle 2 , add 3.5g of potassium hydroxide and 98.4g (1045.4mmol) of methylamine in ethanol (33wt.%), stir, cool to 0°C, and dropwise add 45g (522.7mmol) of succinic dialdehyde. After the dropwise addition was completed, the temperature was naturally raised to room temperature and then to 30° C., and the stirring reaction was continued for 18 hours. After the reaction was finished, 38.7 g of the product N-methylpyrrole was obtained by atmospheric distillation, with a GC content of 97.8% and a yield of 89.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com