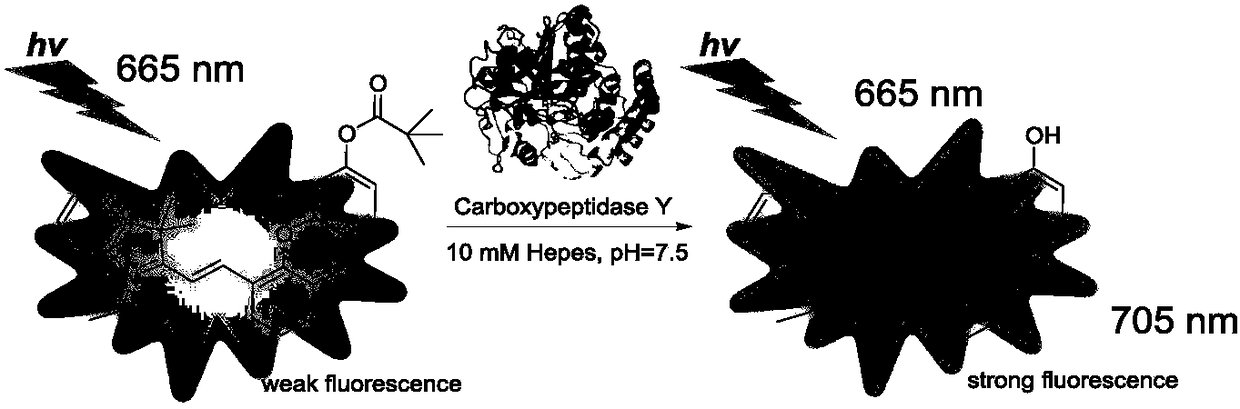
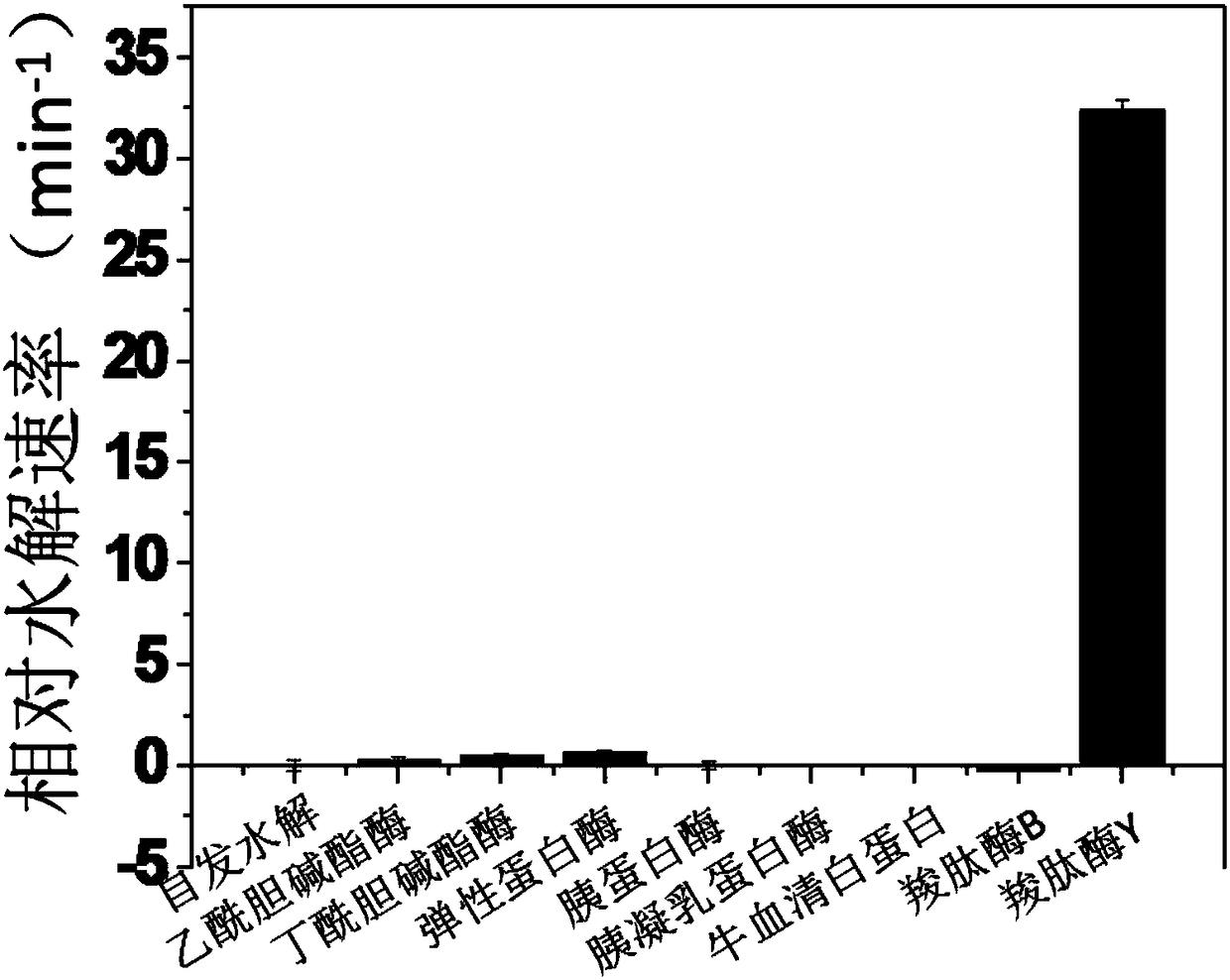
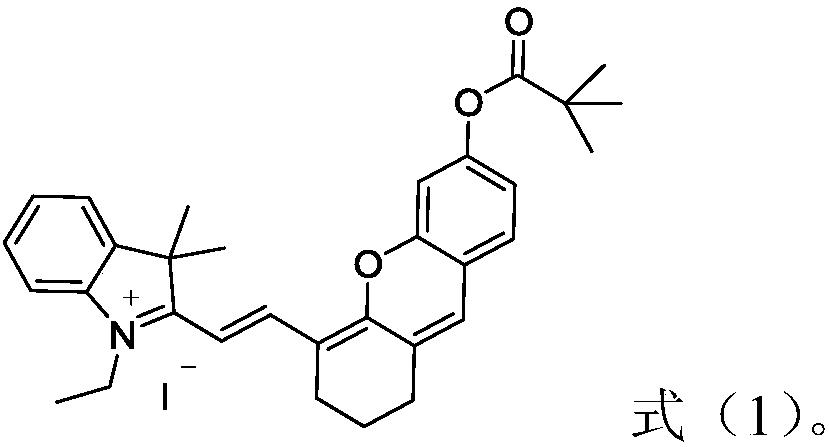
Hemicyanine-based fluorescent probe for detecting carboxypeptidase Y, preparation method and applications thereof
A technology of fluorescent probes and carboxypeptidases, applied in biochemical equipment and methods, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problems of harsh storage and permission conditions, difficult synthesis of polypeptide substrates, and difficulty in substrate synthesis Large and other problems, to achieve the effect of high sensitivity and selectivity, low price and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] In a second aspect, the present invention also provides a method for preparing the above-mentioned semicyanine fluorescent probe, the method comprising: in an organic solvent under the conditions of a nucleophilic reaction and the presence of an acid-binding agent, the compound represented by the formula (2) The structure compound is contacted with pivaloyl chloride;
[0028]
[0029] In the preparation method of the semicyanine fluorescent probe of the present invention, the nucleophilic reaction is the reaction between the hydroxyl group in formula (2) and pivaloyl chloride, and the conditions of the nucleophilic reaction include a temperature of minus 5°C to 30°C, the time is 10-200min, preferably, the condition of the nucleophilic reaction is that the temperature is 0-25°C, and the time is 80-160min.
[0030] In the preparation method of the semicyanine fluorescent probe of the present invention, the contact needs to be carried out in the presence of an acid-bind...
Embodiment 1
[0075] This example is used to illustrate the preparation method of the semicyanine fluorescent probe described in the present invention.
[0076] In an organic solvent (dichloromethane, 20mL), in the presence of an acid-binding agent (triethylamine, 2mmol), the compound (1mmol) represented by formula (2) and pivaloyl chloride (2mmol) were mixed at 5°C, The contact was maintained for 120 minutes to obtain the contacted material. Then, the contacted material was washed with water (50 mL) and saturated saline solution (50 mL) successively to obtain the washed material. The organic phase in the washed material was separated and dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was purified by a silica gel column to obtain 0.27 g of a white solid product.
[0077] 1 H NMR (400MHz, DMSO-d 6 ): \ δ9.14(s,1H),8.60(d,J=8.0Hz,1H),7.79(dd,J 1 =J 2 =4.0Hz,2H),7.60(d,J=8.0Hz,1H),7.50(t,J=6.0Hz,1H),7.44(s,1H),7.38...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com