Preparation method and application of crisaborole topical slow-release liposome
A technology of crisborole and liposome, which is applied in the direction of liposome delivery, medical preparations of non-active ingredients, active ingredients of boron compounds, etc., can solve the problem of inability to get the best of both worlds between the amount of application and the actual curative effect, and the amount of drug transdermal High, loss of active ingredients and other issues, to achieve a stable and controllable release curve, good penetration-promoting effect, and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 150mg of egg yolk lecithin, 50mg of cholesterol and 20mg of crisborole in 4ml of ethanol to form an organic phase. Add 70 mg of polyvinyl alcohol to 10 ml of water to swell overnight as the water phase. Inject the organic phase into the water phase, and homogenize under 800Mpa high pressure for 3-5 times to obtain crisborole liposomes, add 0.1% antioxidant BHT, and fill it in a special spray bottle.
Embodiment 2
[0024] Dissolve 250mg of egg yolk lecithin, 50mg of cholesterol and 20mg of crisborole in 4ml of ethanol to form an organic phase. Add 200mg PEG300 into 10ml water to dissolve, and add 100mg copovidone to swell overnight, as the water phase. Inject the organic phase into the water phase, and homogenize under 800Mpa high pressure for 3-5 times to obtain crisborole liposomes, add 0.1% antioxidant BHT, and fill it in a special spray bottle.
Embodiment 3
[0026] Dissolve 200mg of egg yolk lecithin, 50mg of cholesterol and 20mg of crisborole in 4ml of ethanol to form an organic phase. 200 mg of propylene glycol was added into 10 ml of water to dissolve, and 100 mg of copovidone was added to swell overnight as the water phase. Inject the organic phase into the water phase, and homogenize under 400Mpa high pressure for 3-5 times to obtain crisborole liposomes, add 0.1% vitamin E, and fill it in a special spray bottle.
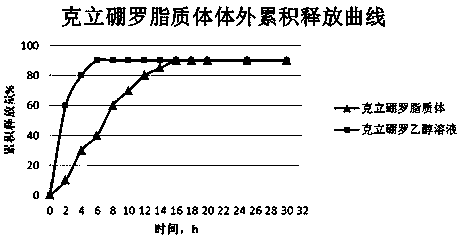
[0027] Such as figure 1 Shown is the in vitro release curve of the local sustained-release liposome prepared by the present invention in a dialysis bag, pH7.4PBS, 500ml, 37°C, and 100rpm / min.
[0028] The application of the treatment method and multiple application methods of the present invention has outstanding substantive features and remarkable progress: by selecting an ionic active agent with a suitable length and controlling the ultrasonic intensity, a uniform particle size dispersion and a stable and contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com