New synthesis method of paliperidone
A technology of paliperidone and compounds, applied in the field of drug synthesis, to achieve high purity, be conducive to industrial production, and avoid the effects of column chromatography purification of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
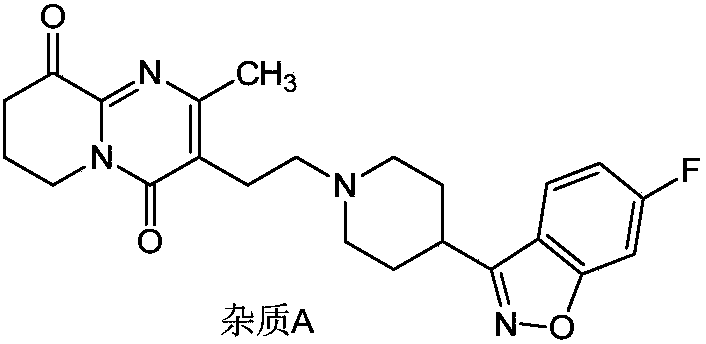
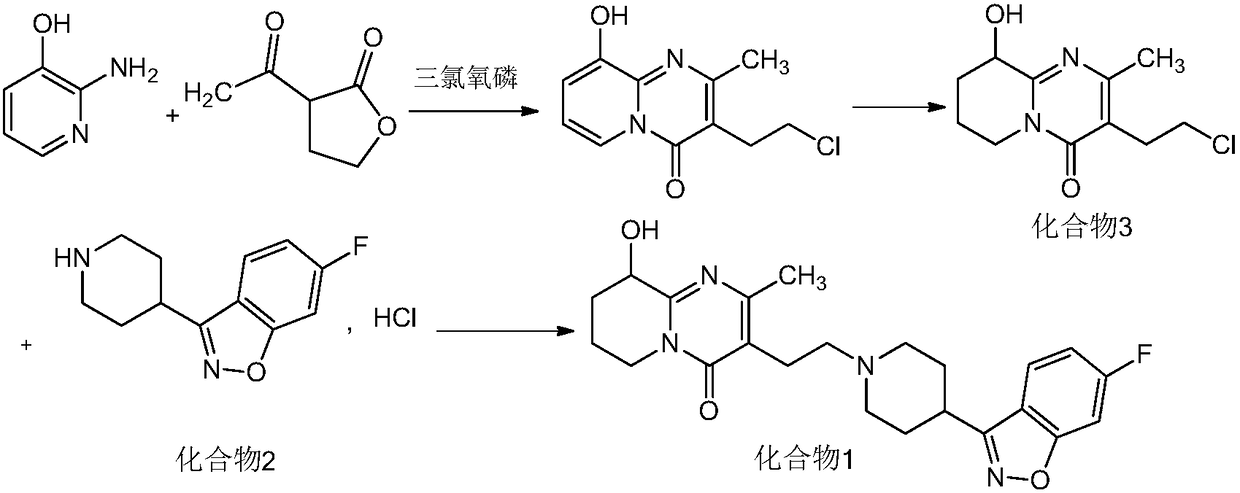
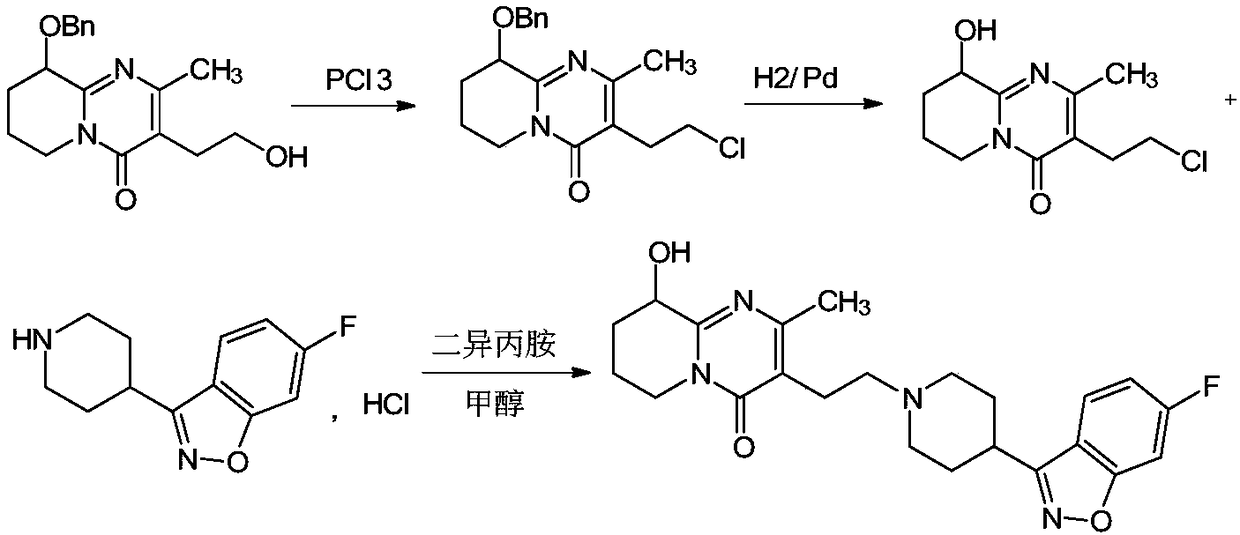
[0026] Add 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride (10.0g, 39mmol), 10ml of diisopropylamine, 3-(2-chloro Ethyl)-6,7,8,9-tetrahydro-9-hydroxyl-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one (9.5g, 39mmol), under nitrogen protection Quickly add 50ml of anhydrous and oxygen-free methanol, stir the reaction solution for 10min, raise the temperature to 60°C and keep it warm for 22-24h. Take an appropriate amount of the reaction solution and send it to HPLC. The content of compound I is 88.79%, and the content of impurity A is 0.16%. The reaction solution is cooled to 5-15°C, filtered, and the obtained filter cake is added to 100ml of water and stirred for 1 hour, filtered, washed with a little ethanol, and dried to obtain Compound I: 14.9 g (35 mmol), molar yield 90%. The sample was sent to HPLC, and the detected compound I content was 98.92%, and the impurity A content was 0.15%.
Embodiment 2
[0028] Add 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride (10.0g, 39mmol), 50ml of methanol, 1.0g of sodium sulfite, and 10ml of diisopropylamine into a 250ml three-necked flask in sequence , the reaction solution was stirred for 10min, and 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidine- 4-ketone (9.5g, 39mmol), the temperature of the reaction solution was raised to 60°C and the reaction was kept for 22-24h. Take an appropriate amount of the reaction solution and send it to HPLC. The content of compound I is 85.72%, and the content of impurity A is 0.03%. The reaction solution is cooled to 0-10°C, filtered, and the obtained filter cake is added to 100ml of water and stirred for 1 hour, filtered, washed with a little ethanol, and dried to obtain Compound I: 14.1 g (33 mmol), molar yield 85%. The sample was sent to HPLC, and the detected compound I content was 98.76%, the impurity A content was 0.03%, and the residue on ignition: ...
Embodiment 3
[0030] Add 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride (10.0g, 39mmol), 50ml of methanol, 0.1g of sodium sulfite, and 10ml of diisopropylamine into a 250ml three-necked flask in sequence , the reaction solution was stirred for 10min, and 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidine- 4-ketone (9.5g, 39mmol), the temperature of the reaction solution was raised to 60°C and the reaction was kept for 22-24h. Take an appropriate amount of the reaction solution and send it to HPLC. The content of compound I is 82.27%, and the content of impurity A is 0.05%. The reaction solution is cooled to 0-10°C, filtered, and the obtained filter cake is added to 100ml of water and stirred for 1 hour, filtered, washed with a little ethanol, and dried to obtain Compound I: 14.2 g (33 mmol), molar yield 85%. The sample was sent to HPLC, and the compound I content was detected as 98.76%, the impurity A content was 0.04%, and the residue on ignitio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com