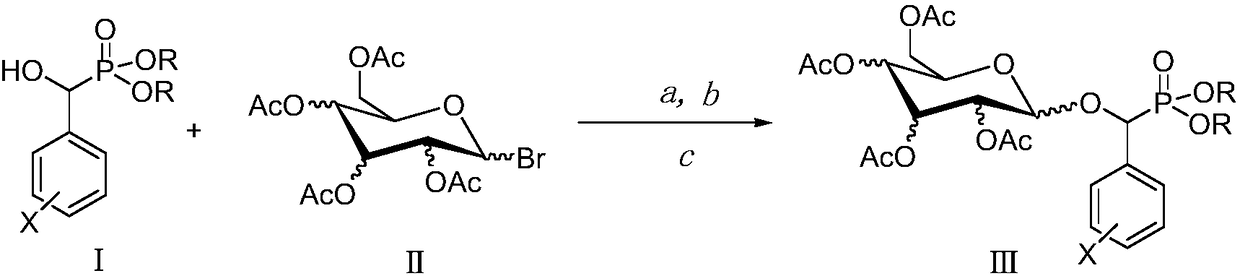Preparation method and anti-tumor application of phosphonate glycoside derivative
A technology of glycoside derivatives and phosphonate esters, which is applied in the field of antitumor drugs and can solve the problems of high toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
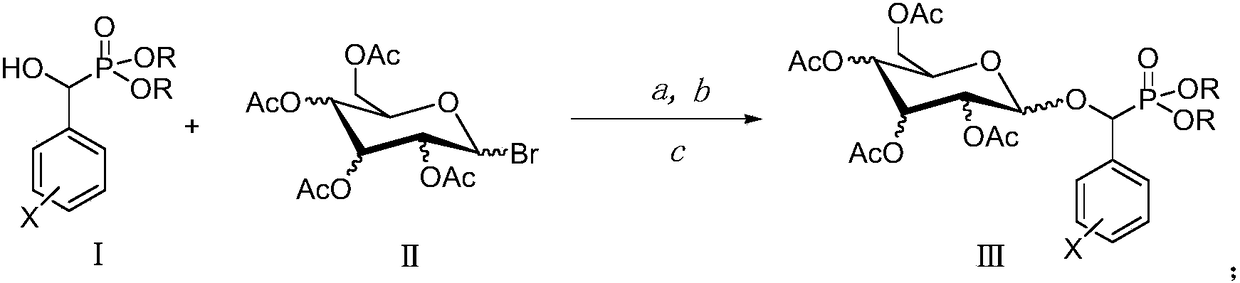
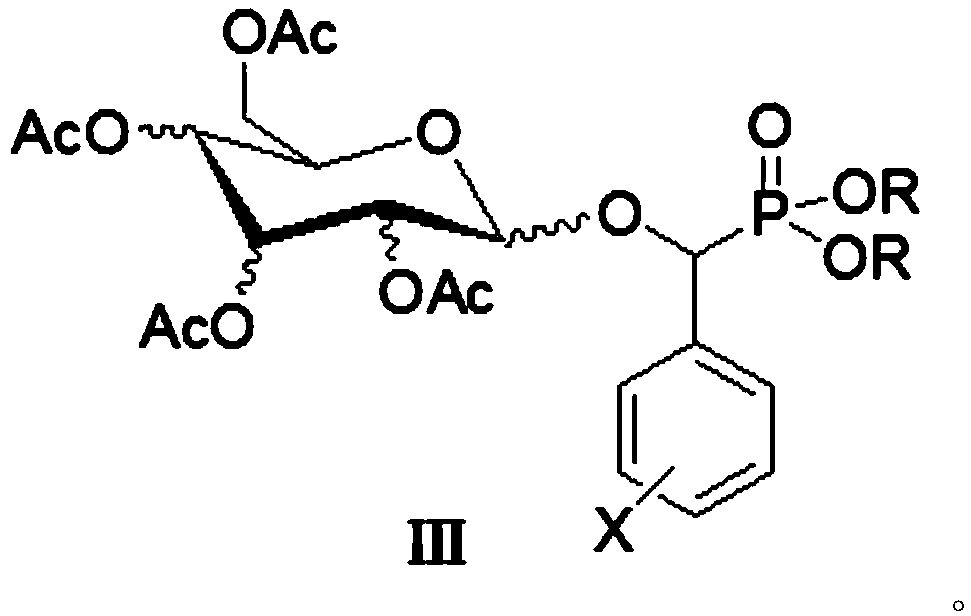
[0011] The preparation method of target phosphonate glycoside derivative (Ⅲ): R is a chain substituent of 1-6 atoms or a cycloalkyl or aromatic hydrocarbon group of 3-6 atoms, X is H or one halogen or multiple halogens or nitro or a chain substituent of 1-3 atoms Or aromatic group, the configuration of the 2,3,4 acetoxy group in the sugar structure can be a horizontal bond (e bond) or a vertical bond (a bond). Its synthetic reaction formula is:
[0012]
[0013] O,O'-diethyl-α-(2-fluorophenyl)-α-(2′,3′,4′,6′-tetra-O-acetyl-β-D-glucopyranosyl) Preparation of Methylphosphonate (Ⅲa)
[0014] Add 2.2mmol of 1-bromo-2′,3′,4′,6′-tetra-O-acetyl-β-D-glucopyranose and 0.6mmol of [Bmim]OH ionic liquid into the three-neck flask, Then add 2.4mmol tetrabutylammonium bromide, O, O'-diethyl-α-(2-fluorophenyl)-α-hydroxymethyl phosphonate 2.0mmol, 50ml chloroform and 50ml purified water. Put it in a microwave synthesizer, react at a microwave power of 900W and a reaction temperature o...
Embodiment 2
[0016] O,O'-diethyl-α-phenyl-α-(2',3',4',6'-tetra-O-acetyl-β-D-galactopyranosyl)-methylphosphine Preparation of acid ester (Ⅲb)
[0017] 2.0mmol of 1-bromo-2′,3′,4′,6′-tetra-O-acetyl-β-D-galactopyranose, [Emim]Cl-AlCl 3 Add 0.6mmol of ionic liquid into the three-neck flask, then add 2.4mmol of tetrabutylammonium bromide and 2.2mmol of O,O'-diethyl-α-phenyl-α-hydroxymethylphosphonate into the reaction flask , 60ml chloroform and 60ml purified water. Place in a microwave synthesizer, react for 70min at a microwave power of 1000W and a reaction temperature of 40°C, stop the reaction, separate the liquid with a separatory funnel, concentrate chloroform to obtain a crude product, separate and purify by column chromatography, and obtain the target compound (Ⅲb) as Yellow viscous liquid, yield 86.9%. 1 H-NMR (400MHz, CDCl 3 )δ:7.12-7.26(m,5H,ArH),5.55(d,1H,J=10.8Hz,OCHO),4.98-5.44(m,4H,4OCH),4.02-4.16(m,7H,PCH+2OCH 2 ),1.90-2.06(m,12H,4COCH 3 ),1.10-1.18(m,6H,2CH 3 ). 13 C-NM...
Embodiment 3
[0019] Antitumor activity test
[0020] The antitumor activity of the prepared phosphonate glycoside derivatives was measured by MTT method with cisplatin as the control. The results showed that the target compounds had proliferation inhibitory activity on human esophageal cancer cell EC-109, such as: compound O,O'-di Ethyl-α-(2-fluorophenyl)-α-(2′,3′,4′,6′-tetra-O-acetyl-β-D-mannopyranosyl)methylphosphonate The IC50 for EC-109 is 8.4±1.3μmol / L, the compound O,O'-diethyl-α-(4-fluorophenyl)-α-(2′,3′,4′,6′-tetra The IC50 of -O-acetyl-β-D-galactopyranosyl)methylphosphonate to EC-109 was 7.5±1.0μmol / L, which was close to that of the control drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com