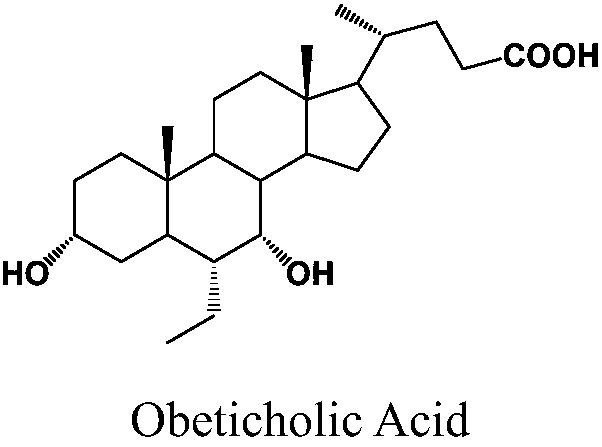
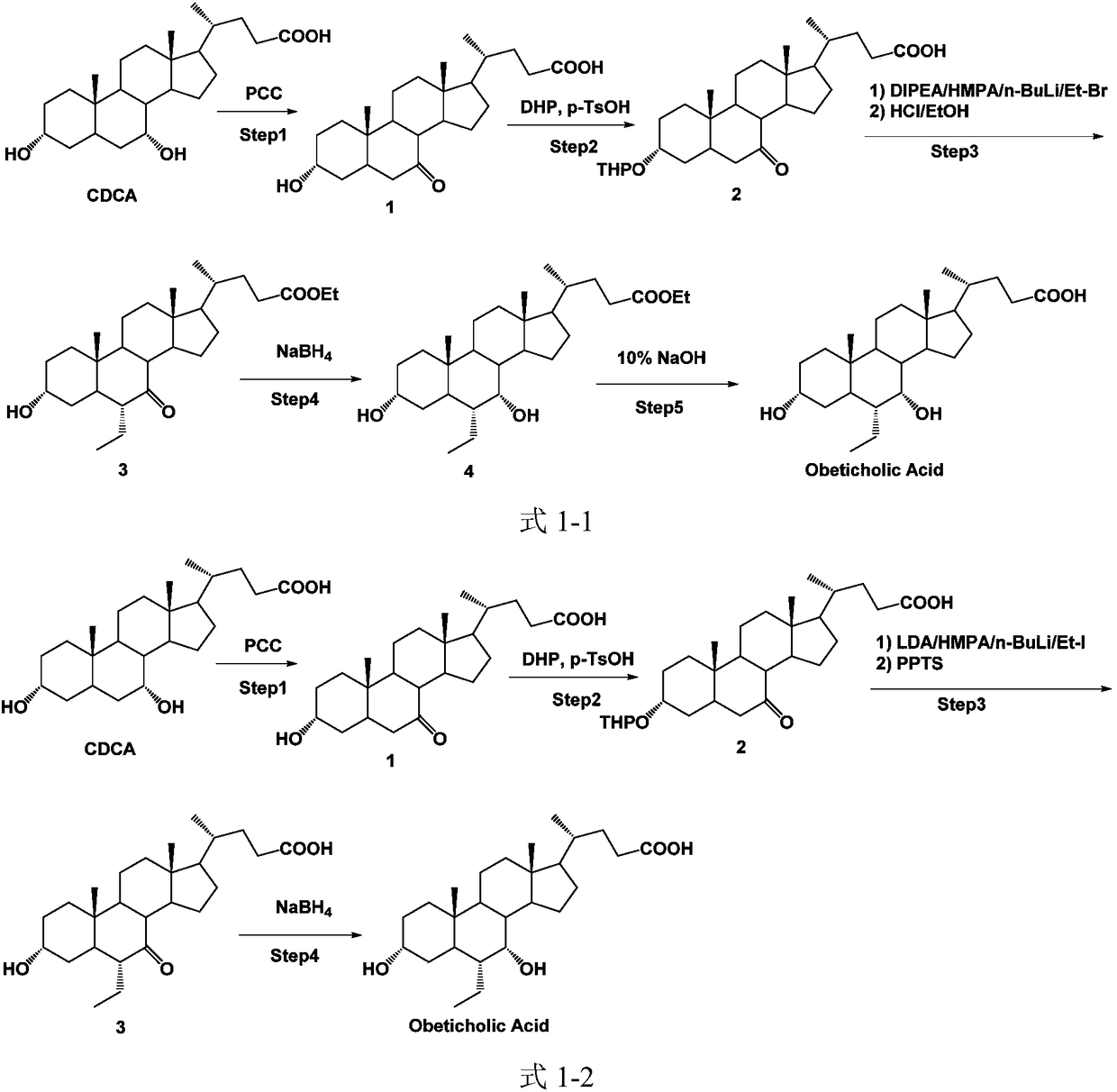
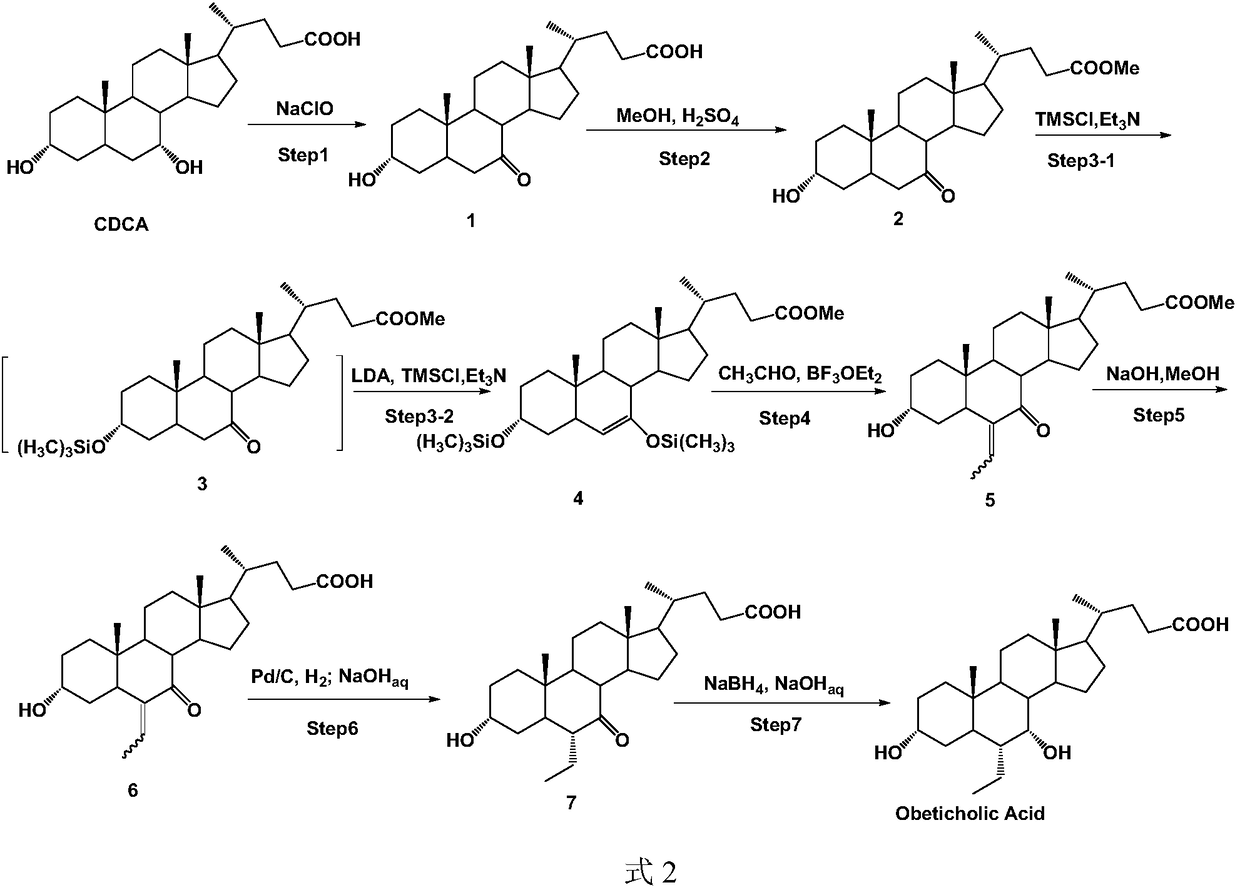
Method of preparing obeticholic acid
A technology of obeticholic acid and intermediates, which is applied in the field of preparation of obeticholic acid intermediates and obeticholic acid, pharmaceutical intermediates, and can solve the problems of increasing the difficulty of feeding and post-processing, increasing production costs, etc. , to achieve the effect of reducing synthesis cost, easy to realize, and good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] carbonyl reduction reaction
[0072] Synthesis of Obeticholic Acid Intermediate 2
[0073]
[0074] 1) In a 1000ml round bottom flask, add 150ml of methanol and 14.0g (9.3mmol) of the starting material in sequence. After the solution is stirred and dissolved and the temperature drops to 0-5°C, add NaBH in batches 4 (1.06g, 27.9mmol); the reaction solution naturally rose to room temperature and continued to react for 8 to 10 hours;
[0075] 2) Reduce the temperature of the reaction solution to 0-5°C, slowly add 150ml of water and 50ml of methanol successively dropwise and continue to stir for 0.5-1 hour;
[0076] 3) The methanol was evaporated under reduced pressure, and then 50 ml of ethyl acetate was added successively to extract the aqueous phase three times;
[0077] 4) After merging the ethyl acetate phases, use anhydrous sodium sulfate to dry the organic phase;
[0078] 5) Ethyl acetate was distilled off under reduced pressure at 45-50°C to obtain 3.94 g of i...
Embodiment 2
[0098] carbonyl reduction reaction
[0099] Synthesis of Obeticholic Acid Intermediate 2
[0100]
[0101] 1) In a 1000ml round bottom flask, add 280ml of ethanol and 12.4g (29.8mmol) of compound 1 in sequence. After the solution is stirred and dissolved and the temperature drops to -5~0℃, add NaBH in batches 4 (3.2g, 83.3mmol), then the reaction solution naturally rose to room temperature and continued to react for 8 to 10 hours;
[0102] 2) Lower the temperature of the reaction solution to -5-0°C, slowly add 20ml of water and 80ml of ethanol dropwise in sequence and continue stirring for 0.5-1 hour;
[0103] 3) ethanol and water are evaporated under reduced pressure;
[0104] 4) Then, 30ml of ethyl acetate was added in turn for beating; after filtration, 12.2g of intermediate 2 was obtained (no need for further treatment, it can be directly put into the follow-up reaction), with a yield of 98.6%.
[0105] Intermediate 2 can be directly put into subsequent reactions wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com