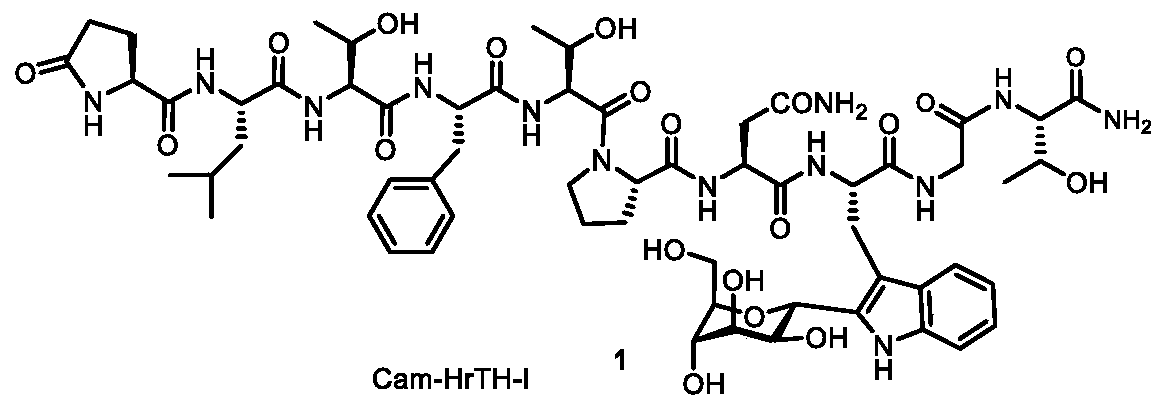
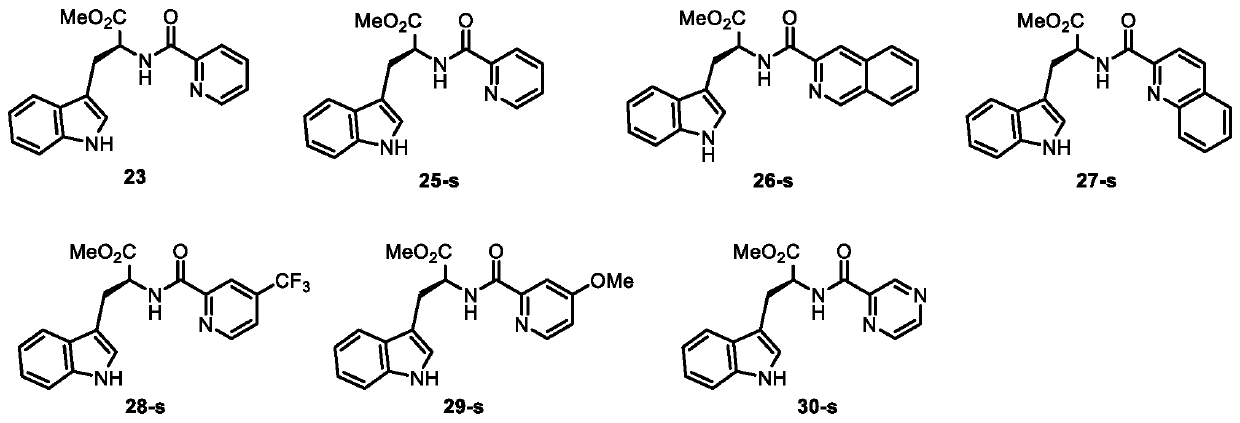
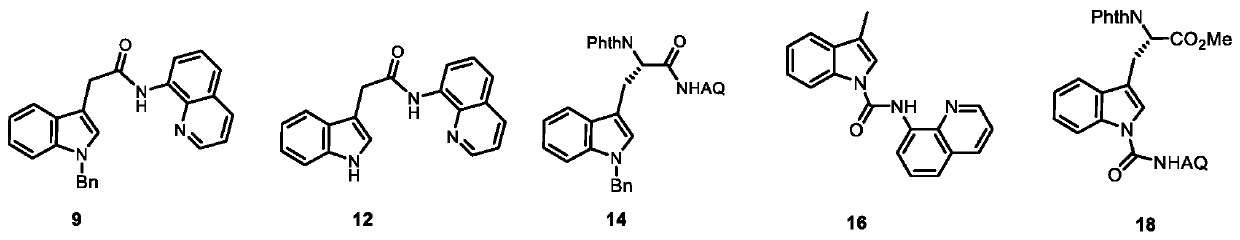
C-alpha-mannosylated tryptophan intermediate as well as preparation method and application thereof
A technology of mannosyl and tryptophan, applied in organic chemistry, bulk chemical production, peptides, etc., can solve the problems of limited reagent application, lengthy synthesis steps, low reaction efficiency, etc., achieve simple and efficient synthesis, and simplify synthesis difficulty , the effect of simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0043] Add amide material (compound 23) (29.2mg, 0.1mmol, 1.0mol / L), chlorosugar (112mg, 0.2mmol, 2.0mol / L), palladium acetate (2.2mg, 0.01mmol, 0.1mol / L), acetyl isoleucine (Ac-Ile-OH) (5.2mg, 0.03mmol, 0.3mol / L), potassium acetate (14.7mg, 0.15mmol, 1.5mol / L), add 1mL solvent , cover the reaction vial cap (without particularly strict anhydrous and oxygen-free conditions), and stir at 110° C. for 12 hours. After the reaction was complete, the reaction bottle was cooled to room temperature, filtered, concentrated, and separated by column chromatography to obtain compounds 24-C and 24-N. The preparation steps of Examples 1-9 are basically the same, the difference lies in the use of different reaction solvents.
[0044]
[0045] The investigation of table 1 reaction solvent
[0046]
[0047] a:NMR Yield
[0048] It can be found from Examples 1-5 that when the reaction solvent is PhMe, the yield of Example 3 is the best.
[0049] Wherein the test data of compound 24-C a...
Embodiment 6-12
[0059] The preparation method of embodiment 6-12 is basically the same as the preparation method of embodiment 3, and the only difference is that the divalent palladium metal catalyst selected in embodiment 6-12 is different, specifically as shown in table 2:
[0060] The screening of table 2 divalent palladium metal catalyst
[0061]
[0062] a:NMR Yield
[0063] Can find by embodiment 6-12, when divalent palladium metal catalyst is Pd(OAc) 2 When, the productive rate of embodiment 3 is the best.
Embodiment 13-17
[0065] The preparation method of Examples 13-17 is basically the same as the preparation method of Example 3, the only difference being that the alkali selected in Examples 13-17 is different, specifically as shown in Table 3
[0066] The investigation of table 3 alkali
[0067]
[0068] a: NMR Yield
[0069] Can find by embodiment 13-17, when alkali is potassium acetate, the productive rate of embodiment 3 is the best.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com