Biaryl pyrrole derived compound and preparation method thereof
An arylpyrrole and compound technology, which is applied in the field of biarylpyrrole derivative compounds and their preparation, can solve problems such as difficulty in synthesis of biaryl compounds, and achieve the advantages of good industrial production, short reaction route, and reduction of intermediate processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
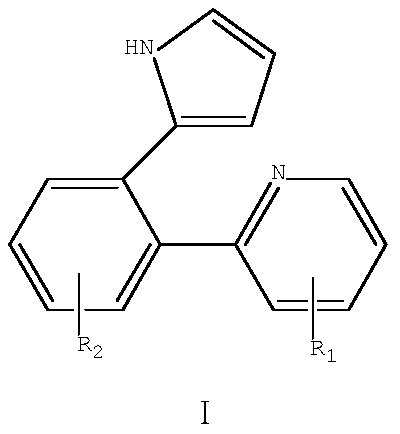
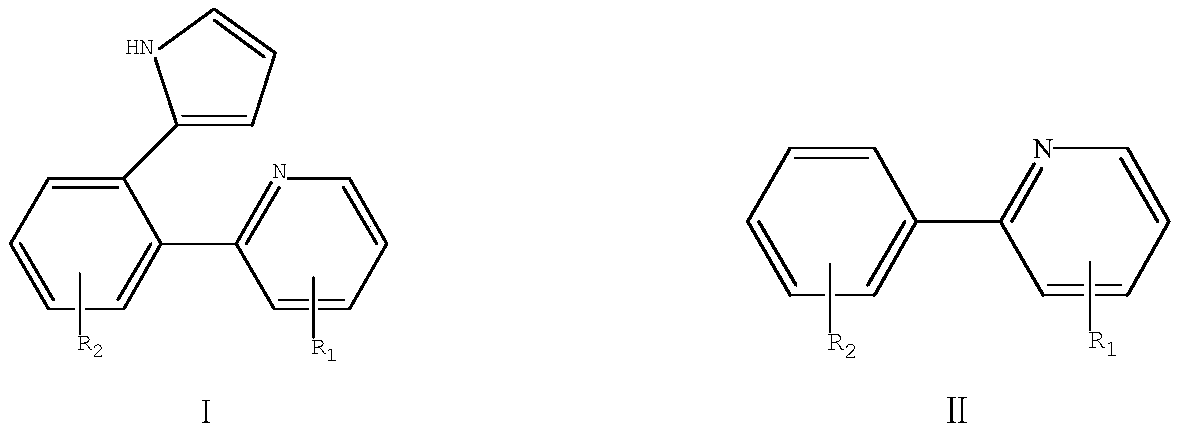
[0036] The structural formula of the biarylpyrrole derivative compound Ia of the present embodiment is as follows:
[0037]
[0038] The structural formula of the corresponding formula II compound (2-phenylpyridine) in the present embodiment is as follows:
[0039]
[0040] In this embodiment, the compound is specifically synthesized by the following method:
[0041] Formula II compound (155mg, 1mmol), pyrrole (670mg, 10mmol), tert-amyl alcohol (20mL), [RuCl 2 (p-cymene)] 2(15mg, 0.025mmol) and copper acetate monohydrate (500mg, 2.5mmol) were put into a clean reaction container, the gas in the container was replaced with oxygen three times and then sealed with a pressure-resistant tetrafluoro bottle stopper, and then the temperature was raised to 105 ° C ~ 110 ℃, in the presence of oxygen, keep warm for oxidative dehydrogenation coupling reaction for 24 hours, after the reaction, slowly cool the reaction solution to room temperature, filter the synthetic solution, rins...
Embodiment 2-8
[0047] Synthesize the corresponding product biarylpyrrole derivative compound Ia according to the operation process of the above-mentioned Example 1, by changing the raw material pyrrole, the catalyst [RuCl 2 (p-cymene)] 2 The consumption and solvent type carry out corresponding concrete implementation, and various consumption ratio situation and corresponding product yield result are as shown in table 1 below:
[0048] Table 1:
[0049]
[0050] For [RuCl in the above table 1 2 (p-cymene)] 2 The consumption is expressed as 30mg (5%mmol) in corresponding embodiment 2 [RuCl 2 (p-cymene)] 2 The dosage of the compound is 30 mg, accounting for 5% mmol of the dosage of the raw material compound of formula II.
[0051] The structural analysis of the product Ia corresponding to Examples 2-8 can be consistent with the corresponding results in Example 1.
Embodiment 9
[0053] The structural formula of the biarylpyrrole derivative compound Ib of the present embodiment is as follows:
[0054]
[0055] The structural formula of the corresponding formula II compound (2-biphenylpyridine) in the present embodiment is as follows:
[0056]
[0057] In this embodiment, the compound is specifically synthesized by the following method:
[0058] Formula II compound (231mg, 1mmol) (2-biphenylpyridine), pyrrole (429mg, 8mmol), tert-butanol (30mL), [RuCl 2 (p-cymene)] 2 (15mg, 0.025mmol) and copper acetate monohydrate (500mg, 2.5mmol) were put into a clean reaction container, the gas in the container was replaced with oxygen three times and then sealed with a pressure-resistant tetrafluoro bottle stopper, and then the temperature was raised to 105 ° C ~ 108 ℃, in the presence of oxygen, keep warm for oxidative dehydrogenation coupling reaction for 22 hours, after the reaction, slowly cool the reaction solution to room temperature, filter the synthe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com