Imide fluoranthene molecular building block as well as preparation and application thereof
A technology of imide fluoranthene and imide, applied in the field of organic photoelectric materials, to achieve the effect of obvious aggregation-induced fluorescence enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
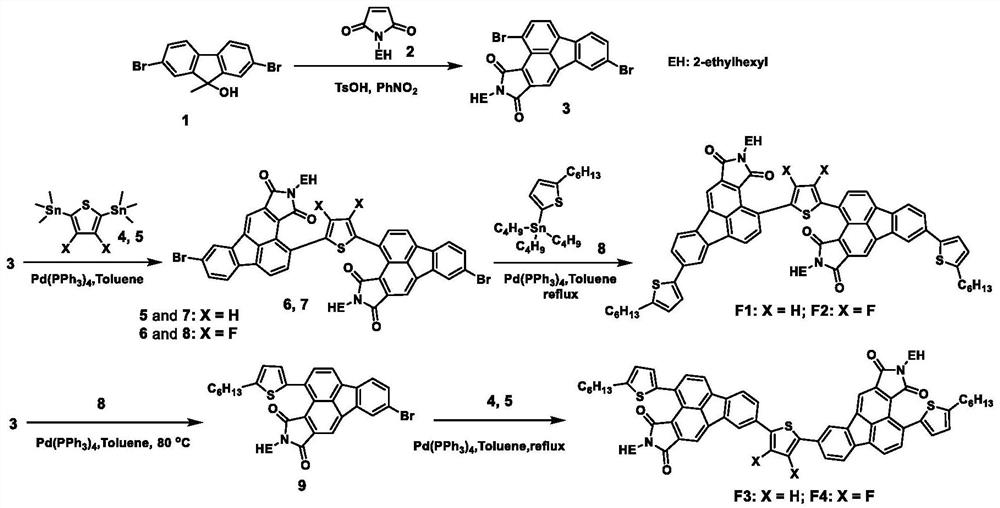
[0078] Synthesis of Compound F1: The synthetic route is as follows figure 1 shown.
[0079] Synthesis of intermediate 3
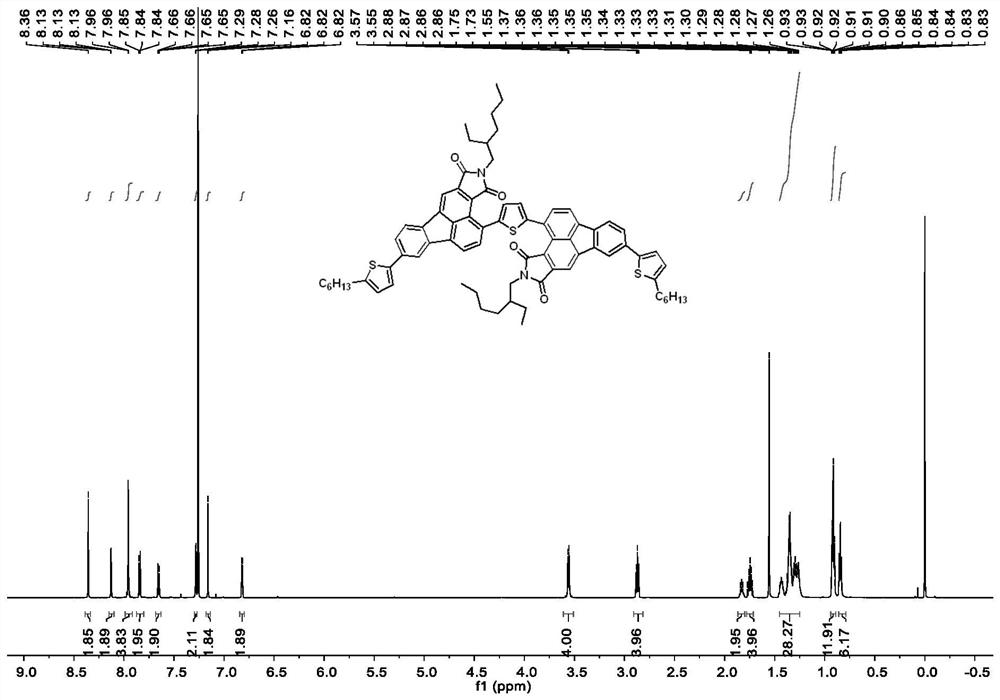
[0080] Compound 1 (2.83g, 8.0mmol), 2-ethylhexyl-maleimide (1.70g, 8.1mmol), p-toluenesulfonic acid (138mg, 0.8mmol) and 15mL of nitrobenzene were added to a 50mL Schlenk bottle , heated to 160-180°C for 6-10h. After the reaction solution was cooled to room temperature, it was added dropwise into methanol solution, and the crude product was obtained by filtration, recrystallized in methanol, filtered, and dried to obtain 2.0 g of a yellow solid with a yield of about 46.3%. 1 H NMR (600MHz, Chloroform-d) δ8.28(s, 1H, ArH), 8.07(d, J=7.4Hz, 1H, ArH), 8.02(d, J=1.7Hz, 1H, ArH), 7.73( d,J=7.4Hz,1H,ArH),7.68(d,J=8.0Hz,1H,ArH),7.57(dd,J=8.0,1.7Hz,1H),3.67(d,J=7.4Hz,2H ,-CH 2 -),1.96–1.87(m,1H,),1.41–1.29(m,8H,-CH 2 -),0.95(t,J=7.4Hz,3H,-CH 3 ),0.90(t,J=7.0Hz,3H,-CH 3 ).
[0081] Synthesis of Intermediate 6
[0082] in N 2 Under conditions, the intermed...
Embodiment 2
[0086] Synthesis of Compound F2: The synthetic route is as figure 1 shown.
[0087] Synthesis of intermediate 7
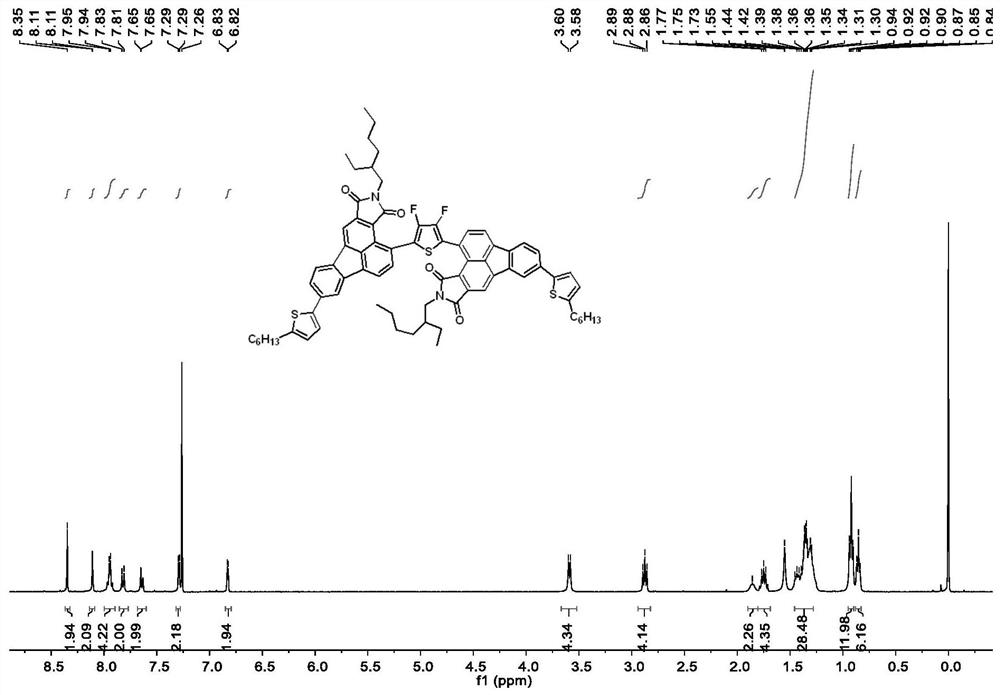
[0088] The synthesis method of intermediate 7 is the same as that of intermediate 6. The starting materials were intermediate 3 (676mg, 1.25mmol), 2,5-di(n-butyltin)-3,4-difluorothiophene (223mg, 0.50mmol) and Pd(PPh 3 ) 4 (58mg, 0.05mmol), the reaction temperature was 90°C, the reaction time was 48h, and the yield was 65.4%. 1 H NMR (600MHz, Chloroform-d)δ8.33(s,2H,ArH),8.10–8.07(m,2H,ArH),8.02(d,J=7.2Hz,2H,ArH),7.97(d,J =7.2Hz,2H,ArH),7.78–7.74(m,2H,ArH),7.61(dd,J=8.0,1.8Hz,2H,ArH),3.60(d,J=7.3Hz,4H,-CH 2 -), 1.85(t, J=6.2Hz, 2H), 1.38–1.29(m, 16H, -CH 2 -),0.92(t,J=7.5Hz,6H,-CH 3 ),0.85(t,J=7.0Hz,6H,-CH 3 ).13C NMR(101MHz,Chloroform-d)δ168.48,167.22,142.57,141.81,139.51,137.86,137.31,136.35,136.13,135.71,132.29,129.07,127.84,125.86,124.72,122.96,122.77,122.21,119.11,113.99 ,42.32,37.24,31.89,31.88,31.55,29.97,29.65,29.60,29.32,29.29,26.43,22.65,22.62,14...
Embodiment 3
[0092] The synthesis of compound F3, synthetic route such as figure 1 shown.
[0093] Synthesis of Intermediate 9
[0094] Compound 3 (676mg, 1.25mmol), Pd(PPh 3 ) 4 (43mg, 0.04mmol) and dry toluene (20mL) were added to a 50mL dry Schlenk bottle, the reaction solution was heated to 70-80°C, and 2,5-bis(tri-n-butyltin)thiophene (571mg, 1.25 mmol) in dry toluene (10 mL), after the dropwise addition was completed, the reaction was continued for 24-30 hours. After cooling, water and dichloromethane were added for extraction, dried, spin-dried and separated through a silica gel column to obtain 248 mg of an orange solid with a yield of 31.6%. 1 H NMR (600MHz, Chloroform-d) δ8.29(s, 1H, ArH), 8.07(d, J=1.7Hz, 1H, ArH), 7.94(d, J=7.2Hz, 1H, ArH), 7.77( d,J=7.2Hz,1H,ArH),7.72(d,J=8.0Hz,1H,ArH),7.58(dd,J=8.0,1.8Hz,1H,ArH),6.91(d,J=3.4Hz ,1H),6.84(d,J=3.5Hz,1H),3.52(d,J=7.3Hz,2H,-CH 2 -),2.90(t,J=7.7Hz,2H,-CH 2 -), 1.75(t, J=7.6Hz, 2H-CH 2 -),1.45(t,J=7.5Hz,2H,-CH 2 -),1.36–1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com