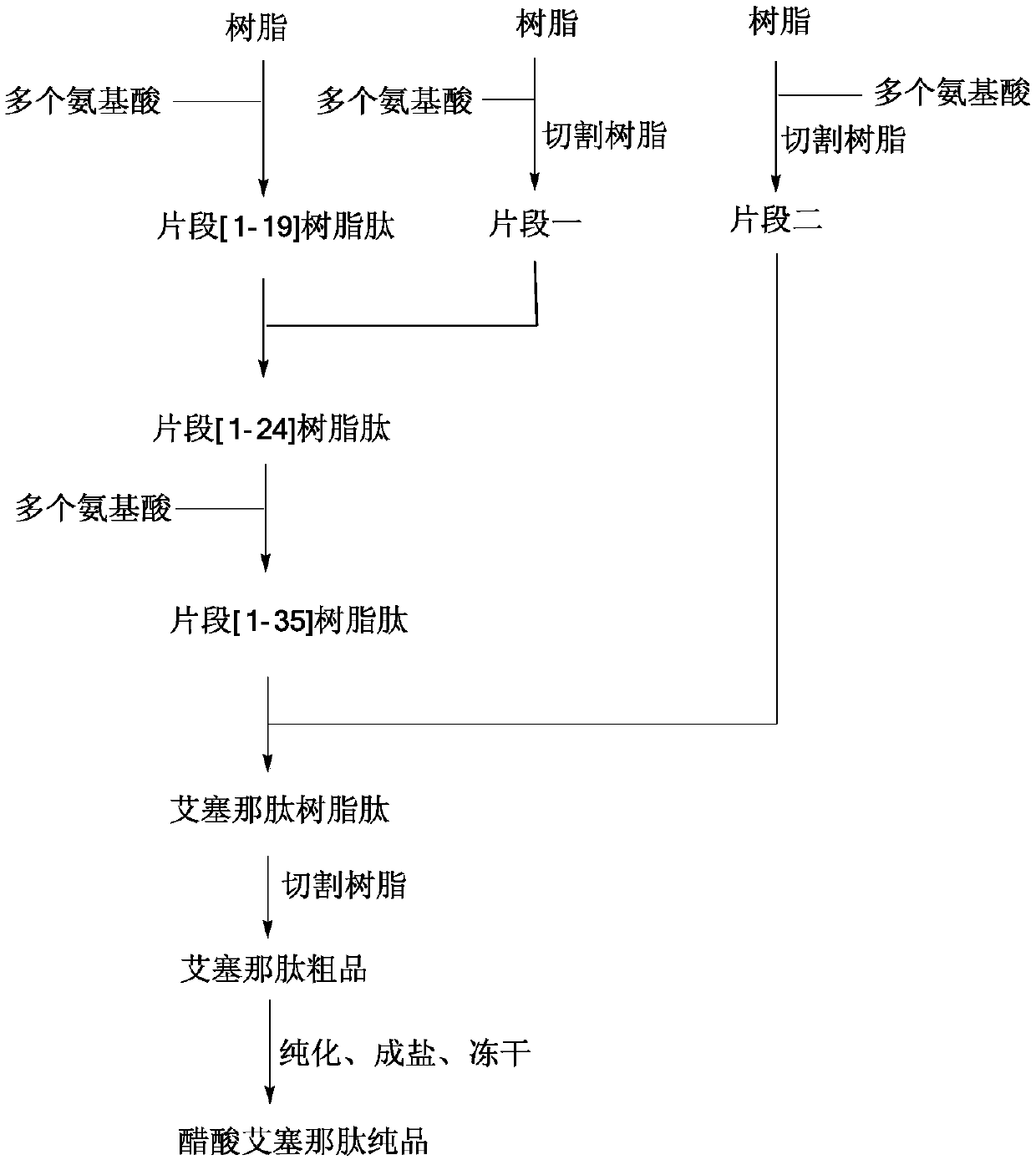
Solid-phase fragment method for the synthesis of exenatide
A technology of exenatide and solid-phase synthesis, which is applied in the direction of peptides, specific peptides, hormone peptides, etc., can solve the problems of low purity and yield, excessive waste liquid, and many impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Synthetic Fragment [1-19] Resin
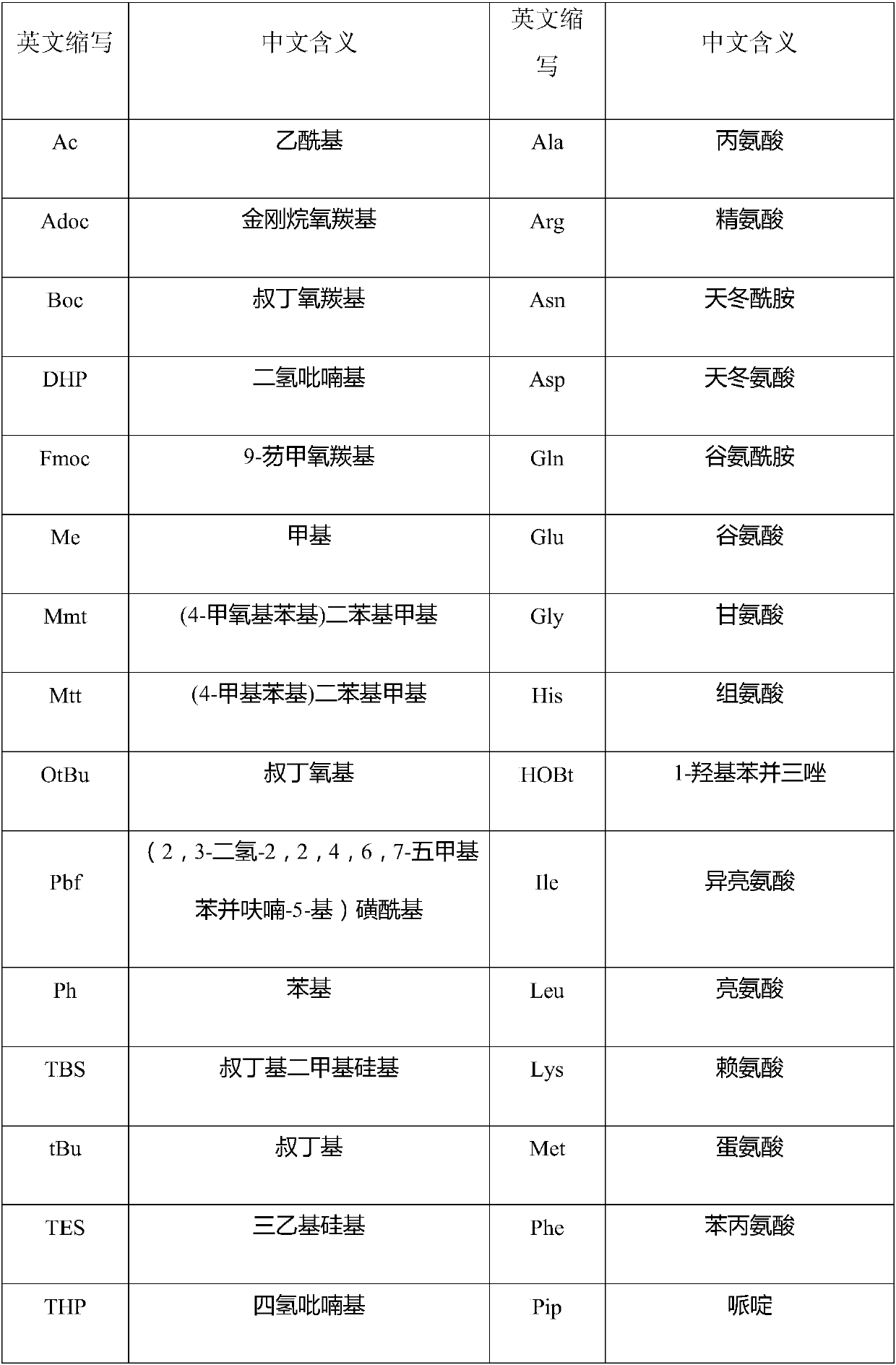
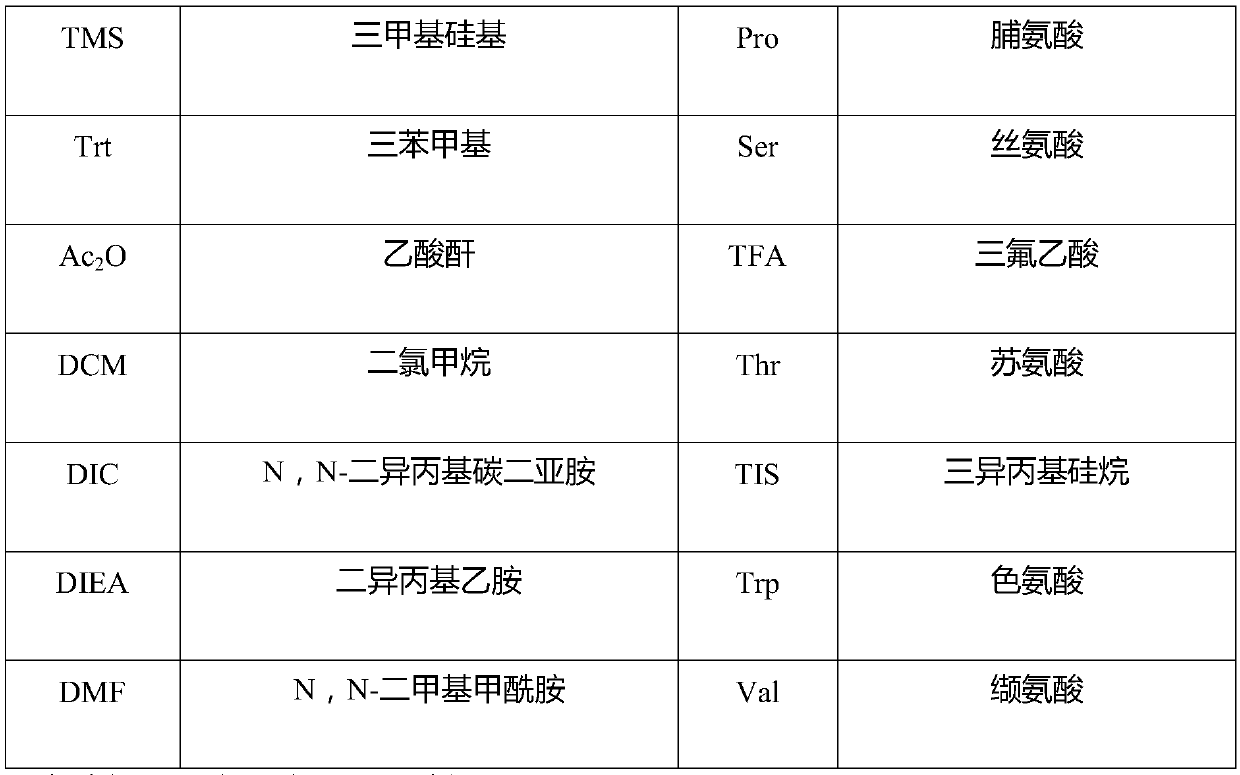
[0069] Fmoc-Rink Amide MBHA resin (degree of substitution 0.312mmol / g, 10g) was put into a solid-phase reaction bottle, swollen with DCM for 30 minutes, washed twice with DMF, and the solution was drained. Add 20% piperidine in DMF solution at room temperature, deprotect the reaction twice, the time is 5min and 15min respectively, drain the solution, wash the resin twice with DMF and IPA alternately, wash twice with DMF, wash twice with DCM, and drain , to obtain a deprotected resin. Add Fmoc-Ser(tBu)-OH (3.59g) and 50mL DMF to the dry reaction flask successively to obtain a solution. Add HOBt (1.52g) and DIC (1.42g) in an ice bath, and stir for 10 minutes to obtain an activated solution. Add the above solution into the solid-phase reaction vial, and stir for 3 hours until the ninhydrin detection coupling reaction ends. Drained, the resin was washed three times with appropriate amount of DMF, and washed three times with ...
Embodiment 2
[0071] Embodiment two: synthetic fragment-resin peptide Weigh 50 g of 2-CTC resin with a substitution degree of 1.1 mmol / g, add it to a solid-phase reaction bottle, and wash it twice with DCM. Add 107.05g of Fmoc-Arg(pbf)-OH and 500mL of DCM to the solid-phase reaction flask in sequence, and after stirring for 5 minutes, slowly add 42.65g of DIEA dropwise, and stir for 2 hours after dropping. Add 100mL methanol and stir for 30min. Wash 3 times with DMF, twice with DCM, twice with methanol, and drain. After vacuum drying, 76.51 g of Fmoc-Arg(pbf)-CTC resin was obtained, and the degree of substitution was measured to be 0.865 mmol / g. Add the resin into the solid phase reactor, add 500mL DMF, stir for 20 minutes, and drain. Add 400 mL of 20% piperidine in DMF, stir at room temperature for 5 min, and drain; add 400 mL of 20% piperidine in DMF, stir for another 15 min at room temperature, and drain. The resin was alternately washed twice with DMF and IPA, twice with DMF, and t...
Embodiment 3
[0072] Embodiment three: synthetic polypeptide fragment one 78.56 g of the resin obtained in Example 2 was added to 1000 mL of 20% TFE in DCM, and stirred for 2 hours. The resin was removed by filtration, and the filtrate was collected and concentrated under reduced pressure to remove the solvent. Add 200mL of DCM to the residue to dissolve, wash with salt water three times, dry the organic phase with anhydrous sodium sulfate for 30min, concentrate under reduced pressure to about 100mL of DCM, add 1000mL of glacial ether for precipitation and crystallization, filter, suck dry, and vacuum dry to obtain 27.36g Fragment 1, yield 86%, purity 97%, mass spectrum 1187.7 (M-H) - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com