Pantoprazole sodium for injection and preparation method thereof
A technology for pantoprazole sodium and injection, which is applied in the field of medicine and can solve the problems of destructive changes in chemical structure, low stability of pantoprazole sodium, poor stability of pantoprazole sodium, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
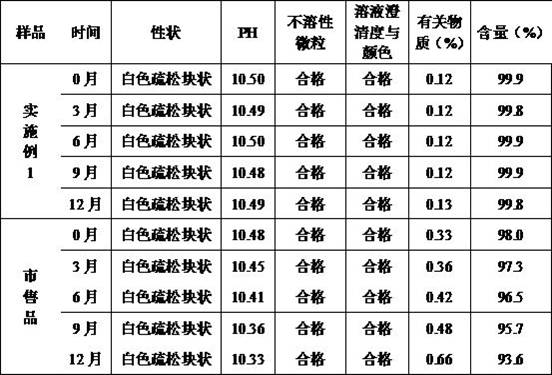
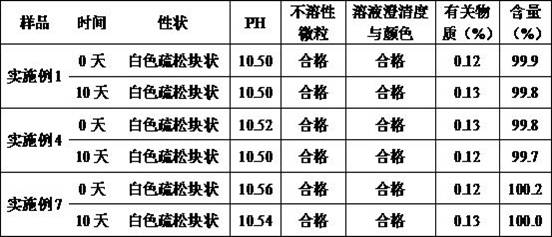
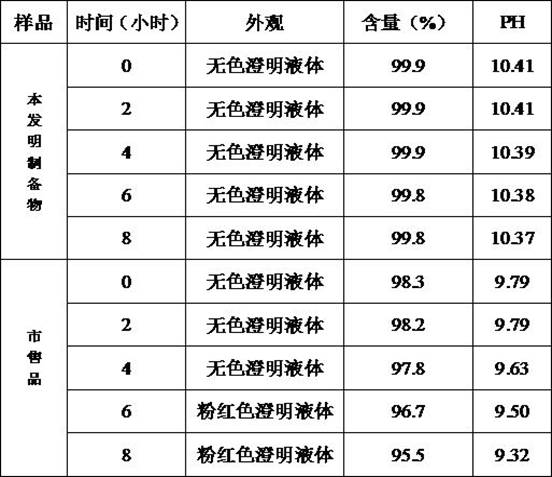
Embodiment 1
[0047] Example 1 Preparation of Pantoprazole Sodium for Injection (Specification: 40mg)
[0048] prescription:
[0049] Pantoprazole sodium: 40g
[0050] Mannitol: 25g
[0051] Edetate disodium: 0.5g
[0052] Water for injection up to 1000ml
[0053] Process:
[0054] 1. According to the prescription, add pantoprazole sodium, mannitol, and edetate disodium into 600ml water for injection and stir to dissolve, add water for injection to 1000ml, and stir well.
[0055] 2. Add 0.10g / 100ml of activated carbon to the solution in step 1, stir for 20 minutes, filter and sterilize with a 0.22μm filter membrane, measure the pH value and content of the filtrate, determine the filling volume (about 1ml) according to the specifications, and divide into half-stoppered Score the liquid.
[0056] 3. Freeze-drying:
[0057] ①Pre-freezing: put the aliquot in a freezer that has been cooled to -33°C in advance, and keep it for 2 hours;
[0058] ②Sublimation: Turn on the vacuum device, a...
Embodiment 2
[0060] Example 2 Preparation of Pantoprazole Sodium for Injection (Specification: 40mg)
[0061] prescription:
[0062] Pantoprazole sodium: 40g
[0063] Mannitol: 25g
[0064] Edetate disodium: 0.5g
[0065] Water for injection up to 1000ml
[0066] Process:
[0067] 1. According to the prescription, add pantoprazole sodium, mannitol, and edetate disodium into 600ml water for injection and stir to dissolve, add water for injection to 1000ml, and stir well.
[0068] 2. Add 0.10g / 100ml of activated carbon to the solution in step 1, stir for 20 minutes, filter and sterilize with a 0.22μm filter membrane, measure the pH value and content of the filtrate, determine the filling volume (about 1ml) according to the specifications, and divide into half-stoppered Score the liquid.
[0069] 3. Freeze-drying:
[0070] ① Pre-freezing: put the aliquot in a freezer that has been cooled to -42°C in advance, and keep it for 3 hours;
[0071] ②Sublimation: Turn on the vacuum device, ad...
Embodiment 3
[0073] Example 3 Preparation of Pantoprazole Sodium for Injection (Specification: 40mg)
[0074] prescription:
[0075] Pantoprazole sodium: 40g
[0076] Mannitol: 25g
[0077] Edetate disodium: 0.5g
[0078] Water for injection up to 1000ml
[0079] Process:
[0080] 1. According to the prescription, add pantoprazole sodium, mannitol, and edetate disodium into 600ml water for injection and stir to dissolve, add water for injection to 1000ml, and stir well.
[0081] 2. Add 0.10g / 100ml of activated carbon to the solution in step 1, stir for 20 minutes, filter and sterilize with a 0.22μm filter membrane, measure the pH value and content of the filtrate, determine the filling volume (about 1ml) according to the specifications, and divide into half-stoppered Score the liquid.
[0082] 3. Freeze-drying:
[0083] ① Pre-freezing: Place the aliquot in a freezer that has been cooled to -37°C in advance and keep it for 22.5 hours;
[0084] ②Sublimation: Turn on the vacuum devi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com