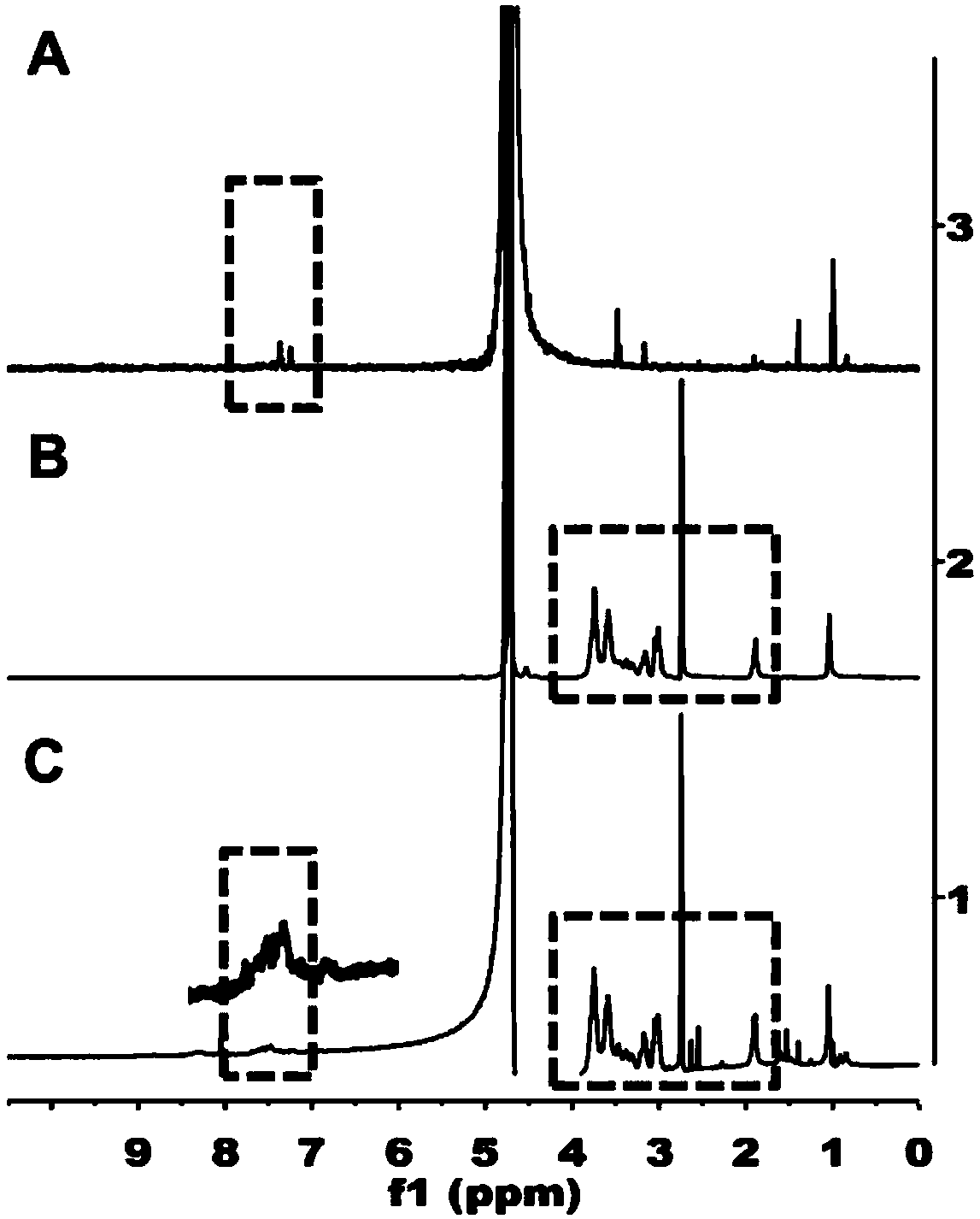
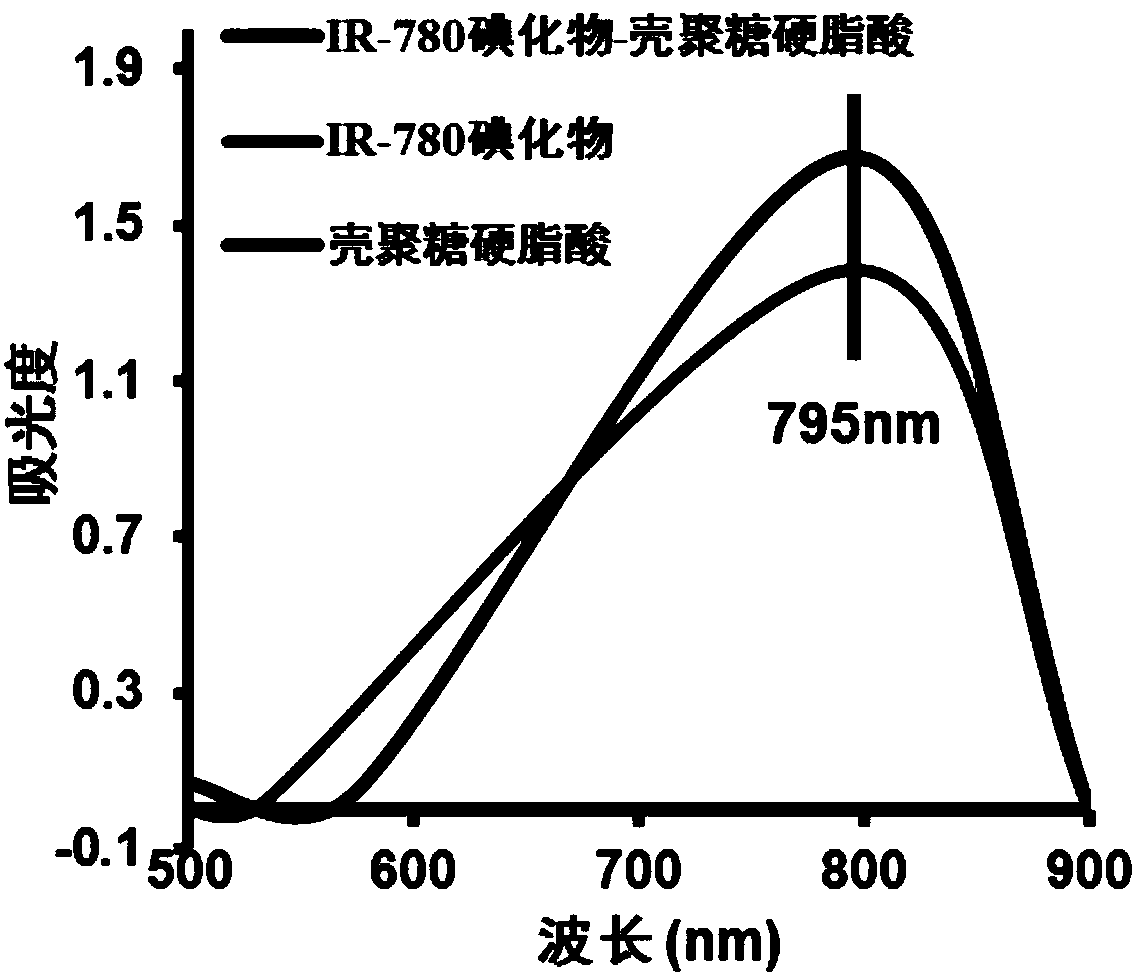
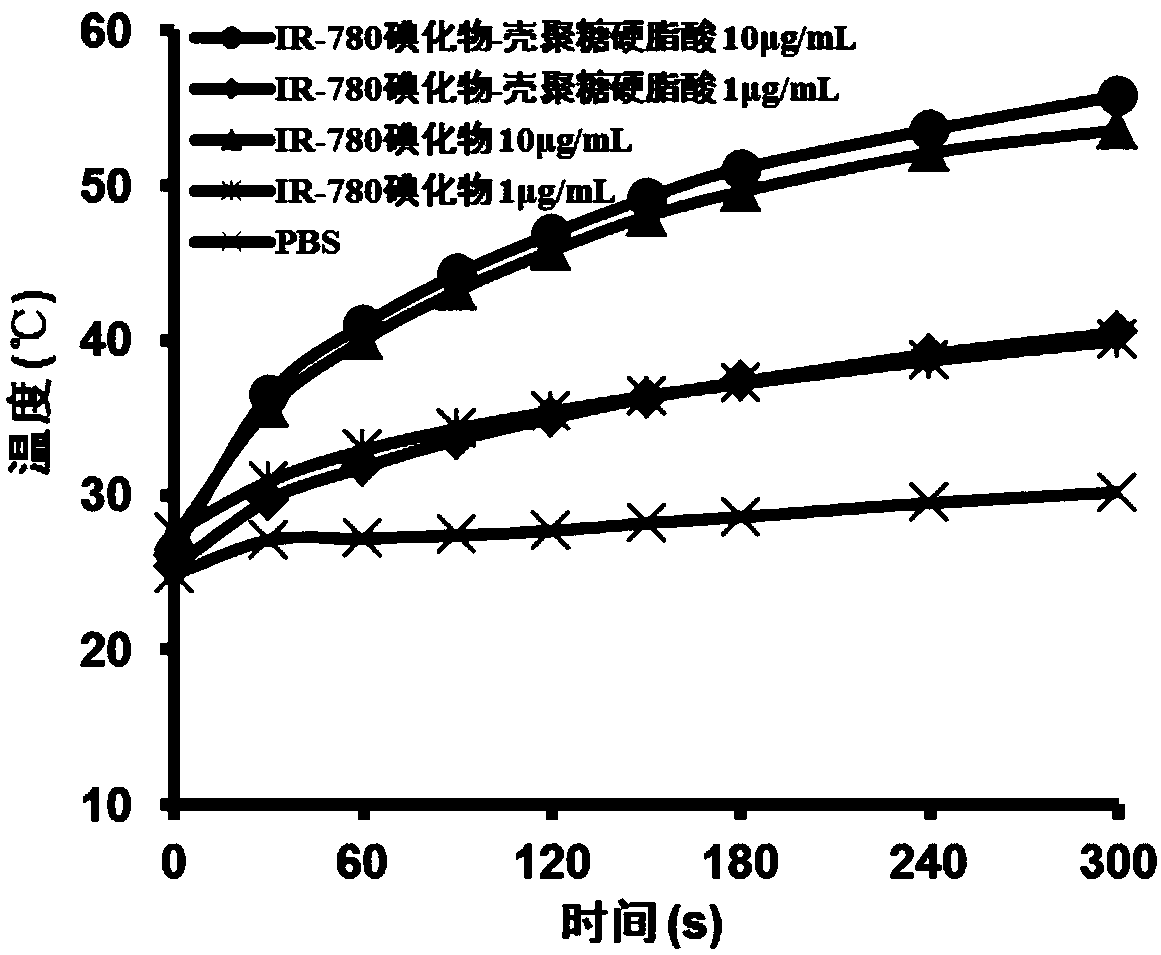
IR-780 iodide-chitosan stearic acid grafted substance as well as preparation and application thereof
A technology of IR-780 and chitosan, which is used in drug combinations, preparations for in vivo tests, and medical preparations with inactive ingredients, etc., can solve problems such as drug leakage, reduce leakage, improve curative effect, and increase concentration. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of low molecular weight chitosan
[0039] Take 50g of chitosan with a molecular weight of 450kDa and a degree of deacetylation of 95%, add it to 1500mL of hydrochloric acid aqueous solution with a volume ratio of 1.2%, and stir for 2 hours at 55°C to fully swell the chitosan, then slowly add 2% chitosan enzyme solution, chitosan enzymolysis reaction is carried out under the condition of 55 ℃, and the degradation degree of chitosan is controlled by gel permeation chromatography. After the reaction is finished, stir at 80°C for 0.5 hour, add 0.3% active carbon in a weight / volume ratio, dilute the reaction solution, filter it with a Buchner funnel, and process the filtrate with a 0.45 μm microporous membrane, freeze-dry to a low Molecular weight chitosan, the weight average molecular weight of the obtained chitosan is 5-20kDa.
[0040] (2) Synthesis of chitosan stearic acid graft
[0041] Take the above-mentioned chitosan with a molecular weight of 5-20 k...
Embodiment 2
[0046] (1) Preparation of low molecular weight chitosan
[0047] Take 50g of chitosan with a molecular weight of 450kDa and a degree of deacetylation of 95%, add it to 1500mL of hydrochloric acid aqueous solution with a volume ratio of 1.2%, and stir for 2 hours at 55°C to fully swell the chitosan, then slowly add 2% chitosan enzyme solution, chitosan enzymolysis reaction is carried out under the condition of 55 ℃, and the degradation degree of chitosan is controlled by gel permeation chromatography. After the reaction is finished, stir at 80°C for 0.5 hour, add 0.3% active carbon in a weight / volume ratio, dilute the reaction solution, filter it with a Buchner funnel, and process the filtrate with a 0.45 μm microporous membrane, freeze-dry to a low Molecular weight chitosan, the weight average molecular weight of the obtained chitosan is 5-20kDa.
[0048] (2) Synthesis of chitosan stearic acid graft
[0049] Take the above-mentioned chitosan with a molecular weight of 5-20 k...
Embodiment 3
[0054] (1) Preparation of low molecular weight chitosan
[0055] Take 50g of chitosan with a molecular weight of 450kDa and a degree of deacetylation of 95%, add it to 1500mL of hydrochloric acid aqueous solution with a volume ratio of 1.2%, and stir for 2 hours at 55°C to fully swell the chitosan, then slowly add 2% chitosan enzyme solution, chitosan enzymolysis reaction is carried out under the condition of 55 ℃, and the degradation degree of chitosan is controlled by gel permeation chromatography. After the reaction is finished, stir at 80°C for 0.5 hour, add 0.3% active carbon in a weight / volume ratio, dilute the reaction solution, filter it with a Buchner funnel, and process the filtrate with a 0.45 μm microporous membrane, freeze-dry to a low Molecular weight chitosan, the weight average molecular weight of the obtained chitosan is 5-20kDa.
[0056] (2) Synthesis of chitosan stearic acid graft
[0057] Take the above-mentioned chitosan with a molecular weight of 5-20 k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com