Method for predicting prognosis and risk of developing hepatocellular carcinoma in liver cirrhosis patient
A technology for hepatocellular carcinoma and liver cirrhosis, used in measuring devices, instruments, scientific instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
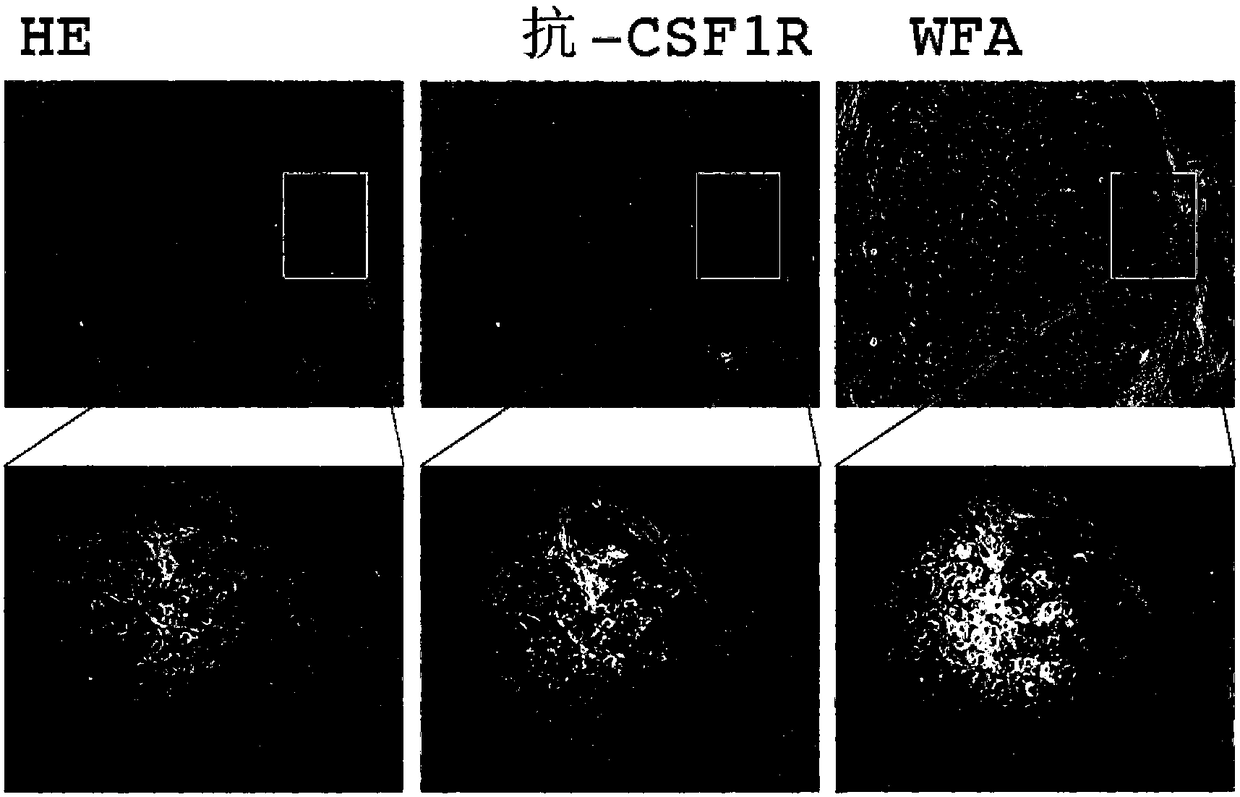
[0266] (Example 1) CSF1R Glycoprotein Analysis Using Immunohistochemical Staining
[0267] Expression studies can be carried out by immunohistochemical staining using lectins or antibodies using specimens (frozen or paraffin-embedded) excised from tissues of patients with various liver diseases, particularly liver cirrhosis or liver cancer. Here, WFA and CSF1R expression in liver cancer tissues were studied using tissue array slides (made from formaldehyde-fixed paraffin-embedded blocks).
[0268] After the tissue array slides were deparaffinized, washed with distilled water, and heated in a microwave oven for 5 minutes in 100 mM citrate buffer (pH 9.0) for antigen activation. Then, endogenous peroxidase inhibition treatment was performed, and blocking buffer (0.2% Tween-20, 5% glycerol, 3% BSA in PBS) was used to block for 20 minutes at room temperature. After washing 3 times in PBS, it was used at 1 μg / mL with primary antibody (anti-CSF1R antibody: C20 clone, Santa Cruz B...
Embodiment 2
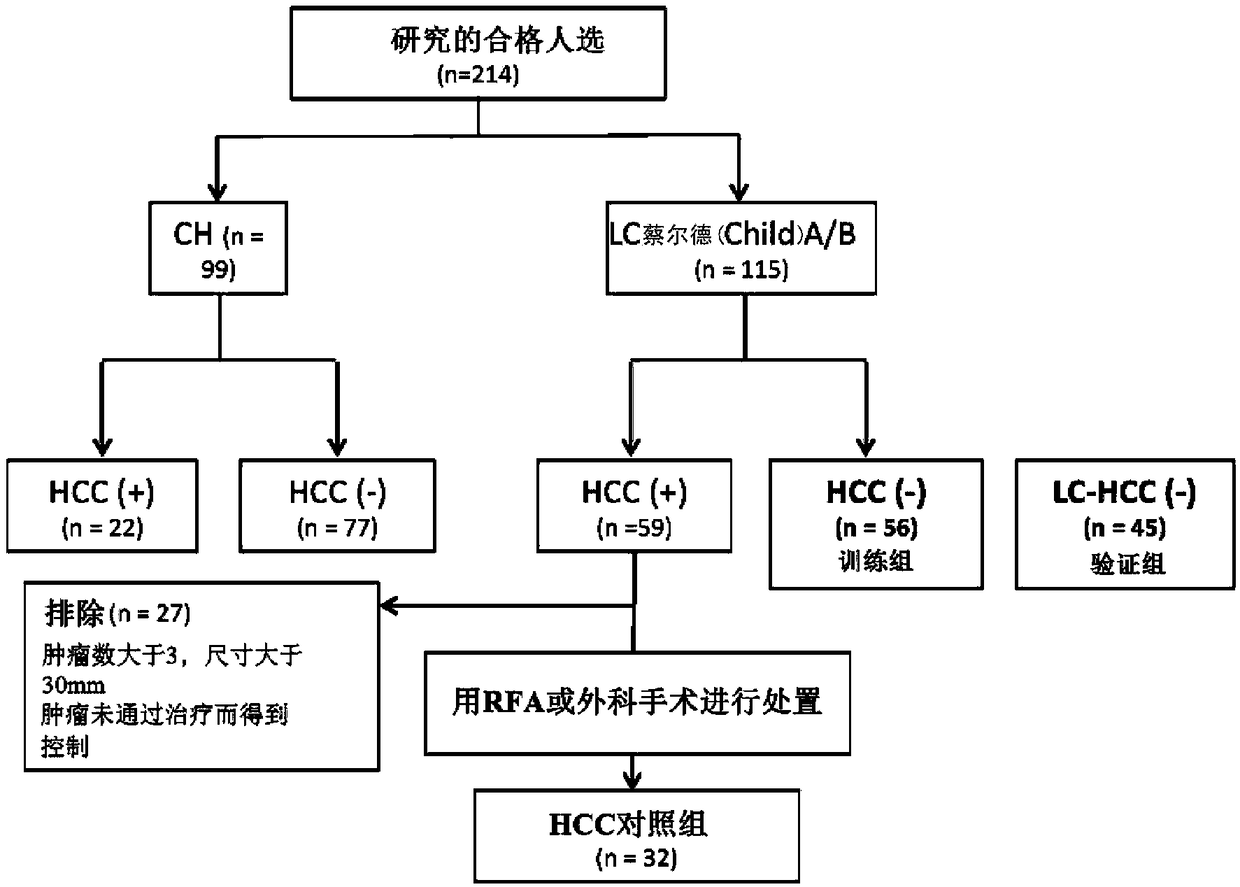
[0270] (Example 2) Clinical trial patients with cirrhosis (LC) and without hepatocellular carcinoma (HCC) (LC(+)HCC (-))s Choice
[0271] Serum obtained from 214 patients with type C chronic liver disease who regularly visited Nagoya City University Hospital between January 1998 and January 2013 was used. HBs antigen-positive patients and patients with other organ malignancies within 3 months after enrollment were excluded. The median observation period was 60 months (1 to 195 months), and patients with Child-pugh classification (Child-Pew classification) C were excluded from the study because they could not accurately evaluate the cancer rate and prognosis due to hospital transfer or the like. Liver fibrosis was evaluated by liver biopsy, ultrasound, and CT. Hepatocellular carcinoma is diagnosed by histological examination or imaging based on the criteria of the American Society of Hepatology. The evaluation of fibrosis grade was judged one by one by two pathologists us...
Embodiment 3
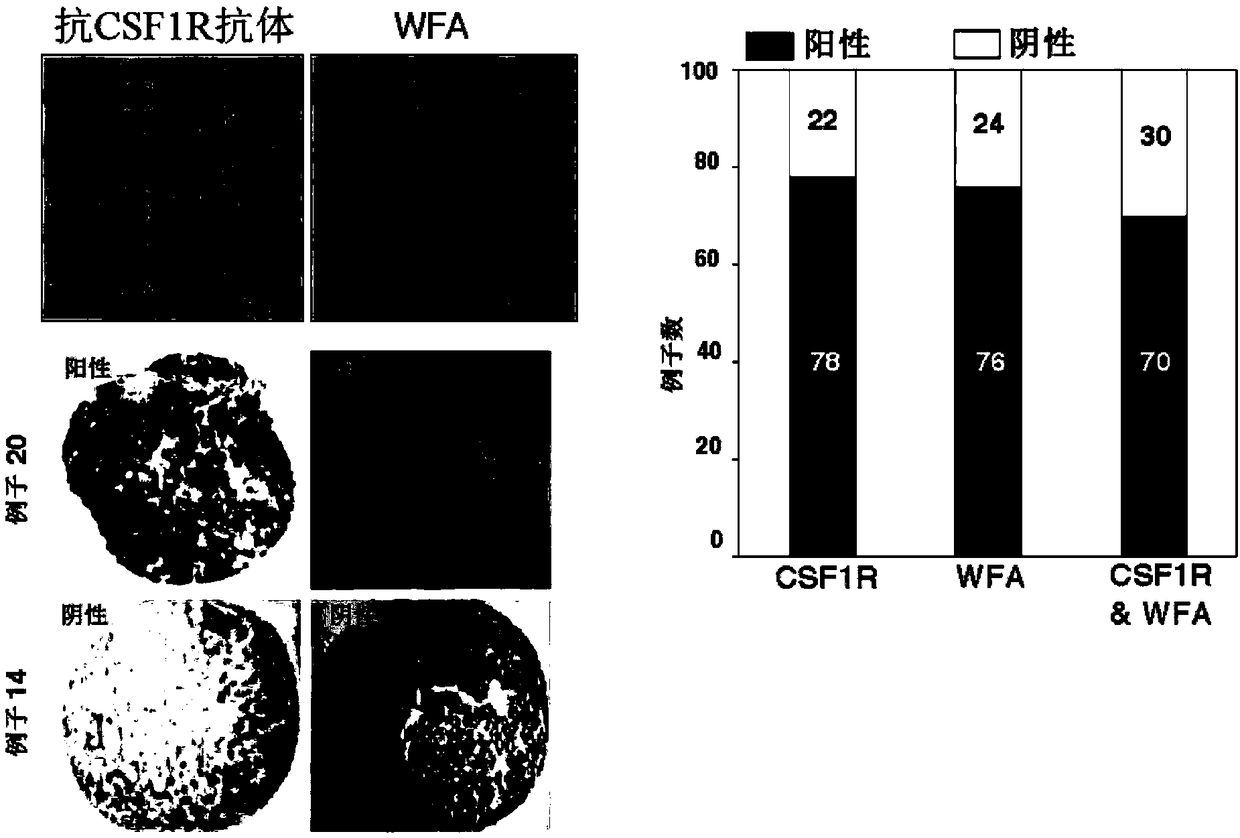
[0279] (Example 3) Indicators for predicting the risk of hepatocellular carcinoma in patients with cirrhosis (LC)
[0280] In this example, an index for predicting the risk of developing hepatocellular carcinoma in liver cirrhosis patients (LC) was studied.
[0281] (3-1) WFA in serum of all patients + - CSF1R concentration and WFA + -CSF1R%
[0282] Measurement of WFA using serum samples of all liver disease patients (214) + - CSF1R and total CSF1R concentrations, results are: Serum WFA in cirrhotic patients (LC) (n=115) compared to hepatitis patients (CH) (n=99) + -CSF1R concentration, total CSF1R concentration were significantly higher [WFA + - CSF1R: 208.9 (34.3-500.9) ng / ml vs. 82.3 (5.0-241.0) ng / ml], [total CSF1R: 845.3 (431.6-1487.5) ng / ml vs. 536.4 (266.3-1357.2) ng / ml].
[0283] Among 115 LC patients (59 HCC, 56 non-HCC): Serum WFA + - CSF1R concentration [208.9 (85.4-500.9) ng / ml vs. 213.0 (34.0-442.0) ng / ml] and total CSF1R concentration [820.9 (431.6-1415....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com