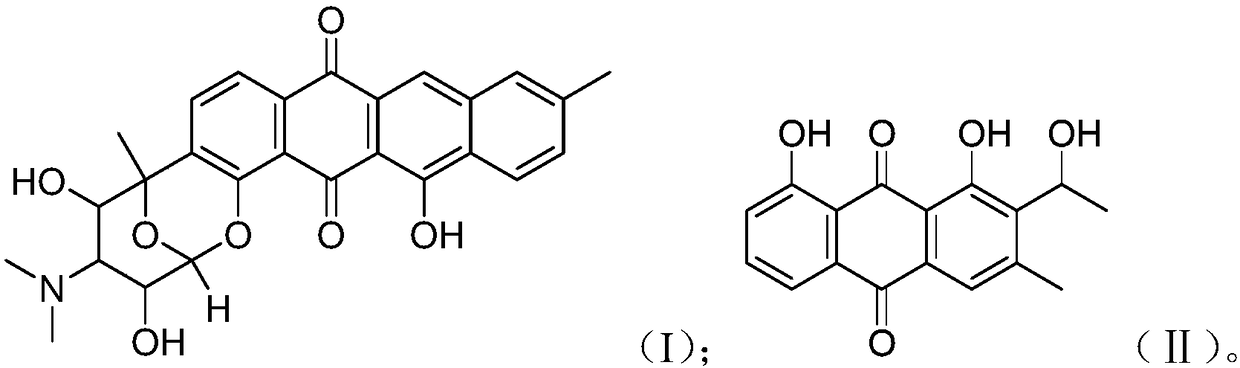
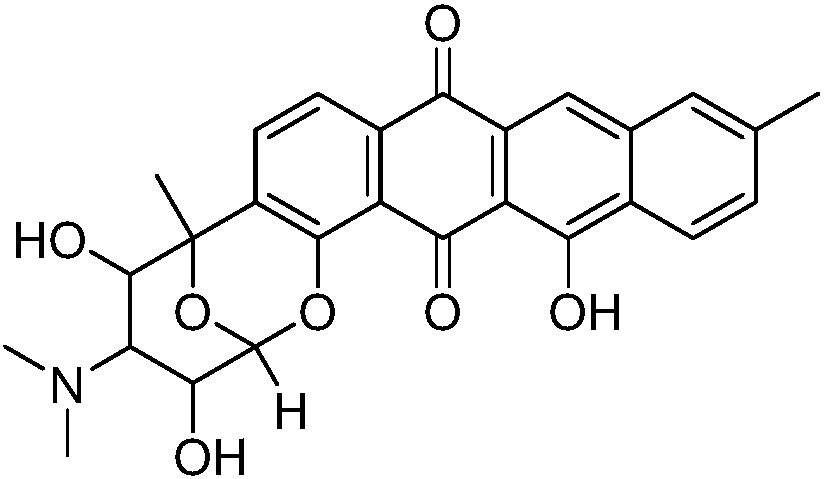
Anthraquinone compound, preparation method thereof, and application to preparation of drug for treating cancer
A technology of anthraquinones and compounds, which is applied in the field of preparation of active compounds from secondary metabolites of actinomycetes, can solve the problems of decreased rate of discovery and waste of manpower, material and financial resources, etc., and achieve the effect of easy cultivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Seed liquid
[0041] Streptomyces rimosus subsp. Paromomycinus NRRL 2455 was inoculated into a 500 mL Erlenmeyer flask containing 250 mL of Gao's No. 1 liquid medium, and cultured with shaking in a shaker (28° C., 180 rpm) for 3-5 days to obtain a seed solution.
[0042] 2. Fermentation
[0043] The above-mentioned seed liquid was inoculated into a rice solid medium in an amount of 10 mL inoculated per bottle, and the fermentation was terminated after standing culture at 28°C for 30 days.
[0044] 3. Rough mention
[0045] Each bottle of solid fermented product was soaked in about 300 mL of EA (ethyl acetate) overnight, the filtrate was collected, and concentrated under reduced pressure to obtain a crude extract (oily extract).
[0046] 4. Compound separation
[0047] (a) The crude extract is purified by silica gel column chromatography, and then is eluted with a gradient of dichloromethane-methanol system with a methanol volume percentage of 0% to 100%; after the components con...
Embodiment 2
[0058] 1. Seed liquid
[0059] Streptomyces rimosus subsp. Paromomycinus NRRL 2455 was inoculated into a 500 mL Erlenmeyer flask containing 250 mL of Gao's No. 1 liquid medium, and cultured with shaking in a shaker (28° C., 180 rpm) for 3-5 days to obtain a seed solution.
[0060] 2. Fermentation
[0061] The above-mentioned seed liquid was inoculated into a rice solid medium in an amount of 10 mL inoculated per bottle, and the fermentation was terminated after standing culture at 28°C for 30 days.
[0062] 3. Rough mention
[0063] Each bottle of solid fermented product was soaked in about 300 mL of EA (ethyl acetate) overnight, the filtrate was collected, and concentrated under reduced pressure to obtain a crude extract (oily extract).
[0064] 4. Compound separation
[0065] (a) The crude extract is purified by silica gel column chromatography, and then is eluted with a gradient of dichloromethane-methanol system with a methanol volume percentage of 0% to 100%; after the components con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com