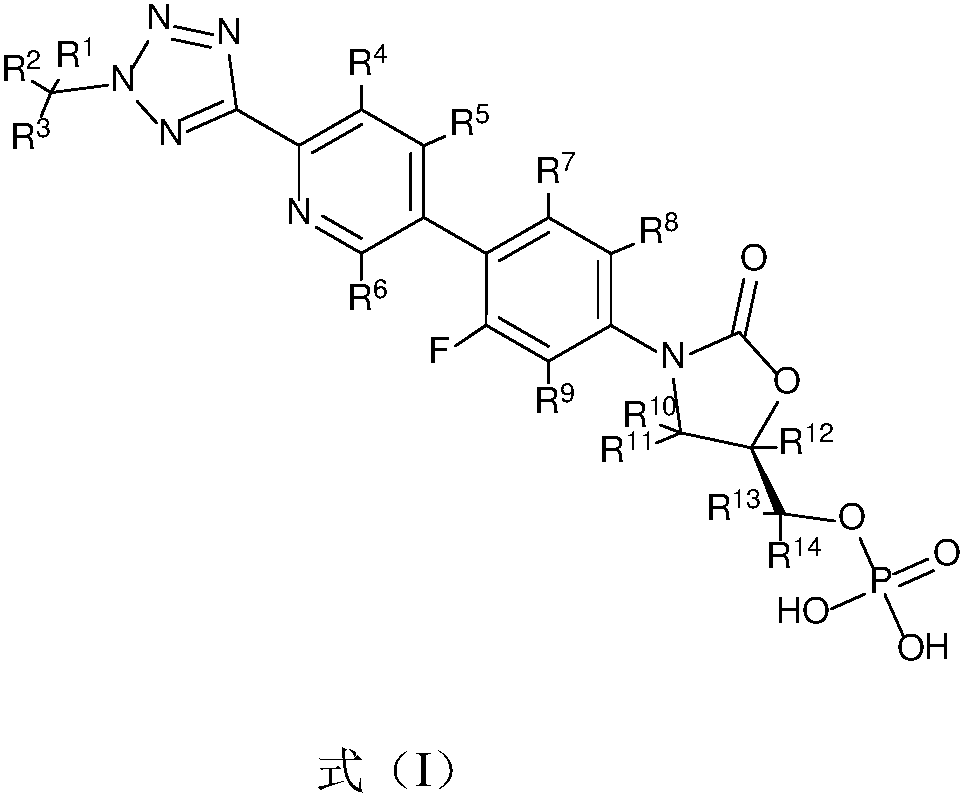
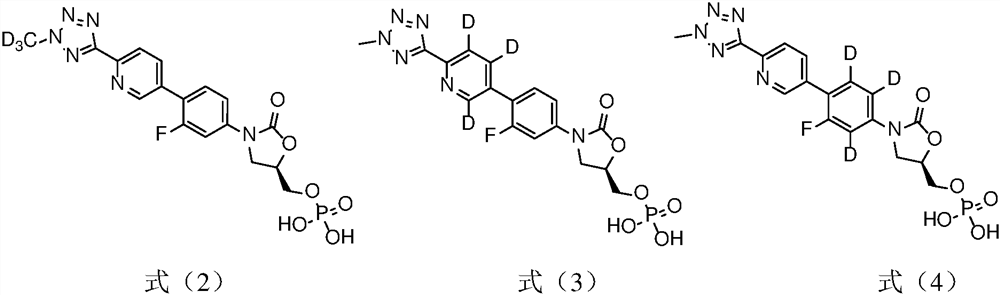
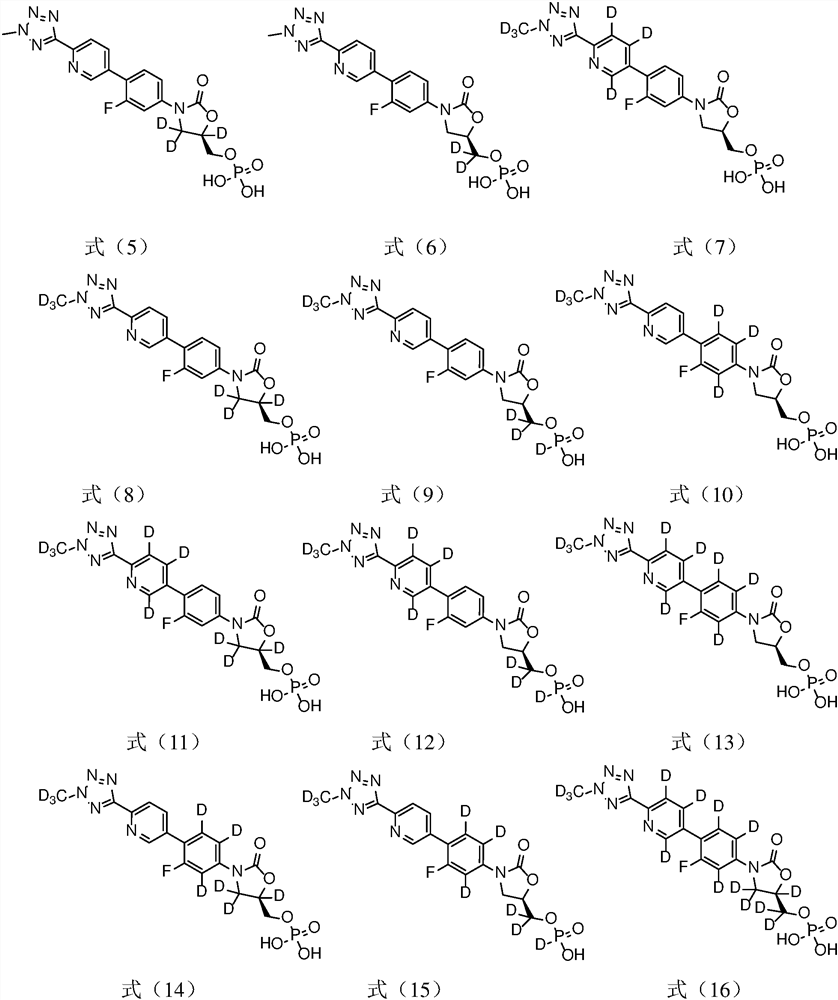
Substituted oxazolidinone compounds and applications thereof
A compound and composition technology, applied in the field of medicine, can solve the problems of incurable infection and drug resistance, and achieve the effects of good pharmacokinetic parameter characteristics, changing dosage, and improving applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of (R)-3-(4-(2-(2-d3-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxy Methyloxazolidin-2-one phosphate (compound T-1)
[0040]
[0041] Concrete synthetic steps are as follows:
[0042]
[0043] Step 1 Synthesis of 2-(2-d3-methyltetrazol-5-yl)-5-bromopyridine (Compound 2).
[0044] 5-bromo-2-(2H-tetrazol-5-yl)pyridine (0.5g, 2.2mmol) and K 2 CO 3 (0.61g, 4.42mmol) was dissolved in 10mL DMF, and deuterated methyl iodide (0.42g, 2.88mmol) was slowly added dropwise under ice bath. After dropping, the mixture was stirred under ice bath for 1 hour, and TLC detected that the starting material disappeared. Add 40mL of water to the reaction solution, extract with ethyl acetate, wash the organic phase with 20mL of saturated brine, dry over anhydrous sodium sulfate, concentrate, and column chromatography to obtain the oily 2-(2-(methyl-d3)tetrazole -5-yl)-5-bromopyridine (compound 2) 0.27g, yield 52.5%. 1 H NMR (300MHz, CDCl 3 ) 8.8...
Embodiment 2
[0051] Example 2 Preparation of (R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl-3,4,6-d3)-3-fluorobenzene Base) -5-hydroxymethyloxazolidin-2-one phosphate (compound T-2)
[0052]
[0053] Concrete synthetic steps are as follows:
[0054]
[0055] Step 1 Synthesis of 2-(2-methyltetrazol-5-yl)-5-bromopyridine (Compound 6).
[0056] 5-bromo-2-(2H-tetrazol-5-yl)pyridine (0.5g, 2.2mmol) and K 2 CO 3 (0.61g, 4.42mmol) was dissolved in 10mL of DMF, and iodomethane (0.41g, 2.88mmol) was slowly added dropwise under ice-cooling. After dropping, the mixture was stirred under ice bath for 1 hour, and TLC detected that the starting material disappeared. 40mL of water was added to the reaction solution, extracted with ethyl acetate, the organic phase was washed with 20mL of saturated brine, dried over anhydrous sodium sulfate, concentrated, and column chromatography gave 2-(2-methyltetrazol-5-yl )-5-bromopyridine 0.28g, yield 52.7%. 1 HNMR (300MHz, CDCl 3 ) 8.83 (d, J=2.3Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com