Pyridine compound containing adamantane substituent groups, and applications thereof in preparation of anti-tumor drugs
A technology of compounds and pyridines, applied in the field of chemical medicine, can solve problems such as the influence of normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
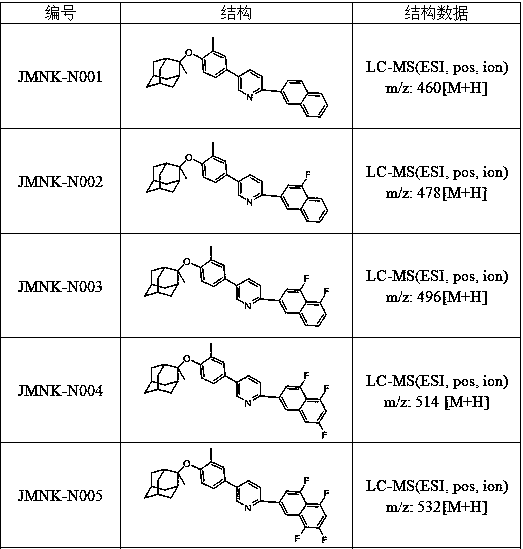
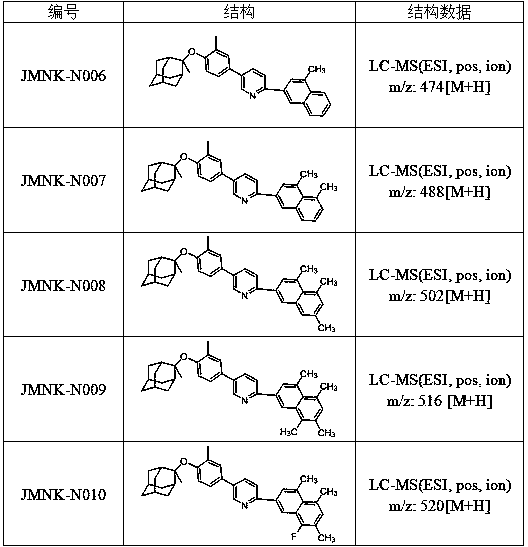
[0024] Example 1: 2-(1-cyclohexylvinyl)-5-(3-methyl-4-(((1R, 3R, 5R, 7R)-2-methyladamantan-2-yl)oxy ) Synthesis of phenyl) pyridine
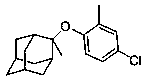
[0025] 1.1 Synthesis of (1r, 3r, 5r, 7r)-2-(4-chloro-2-methylphenoxy)-2-methyladamantane
[0026]
[0027] To a solution of (1R,3R,5R,7R)-2-methyladamant-2-ol (Compound 1) (111.4mg, 0.67mmol) dissolved in DMF (6.7mL) at 0-5°C Add 60% NaH (34.8 mg, 0.87 mmol) by mass fraction, then stir the above solution at room temperature for 30 minutes, add 1,4-dichloro-2-methylbenzene (compound 2) (107.9 mg, 0.67 mmol) And the reaction was stirred for 1 hour. The obtained crude product was diluted with EtOAc. Transfer to a separatory funnel, separate the layers and separate the organic layer, wash the organic layer with water (30 mL), brine, wash over Na 2 SO 4 Drying, filtration and concentration under reduced pressure gave (1r,3r,5r,7r)-2-(4-chloro-2-methylphenoxy)-2-methyladamantane (compound 3), 146.1 mg, yielding The rate is 75%. The crude pro...
Embodiment 2
[0034] Example 2: 2-(3-fluoro-naphthalen-2-yl)-5-(3-methyl-4-(((1R, 3R, 5R, 7R)-2-methyladamantan-2-yl )Synthesis of oxy)phenyl)pyridine
[0035]
[0036] 3-fluoro-naphthalene-2-boronic acid (0.375mmol), compound 4 (137.97mg, 0.375mmol), Na 2 CO 3 (119.8mg, 1.13mmol), DME (0.61mL) and H 2 O (0.15 mL) was added to a 5 mL microwave vial. vial with N 2 Degas for 11 minutes, then add PdCl 2 (dppf)CH 2 Cl 2 (33.1 mg, 0.045 mmol) adduct. The reaction mixture was heated at 120 °C for 50 minutes by microwave irradiation. The resulting mixture was diluted with ethyl acetate and filtered through celite, then concentrated in vacuo. Purification by flash chromatography using 0-100% ethyl acetate / heptane as eluent gave 2-(3-fluoro-naphthalen-2-yl)-5-(3-methyl-4) as a bright yellow powder -(((1R,3R,5R,7R)-2-methyladamantan-2-yl)oxy)phenyl)pyridine, 146.7 mg, 82% yield. LC-MS (ESI, pos, ion) m / z: 478[M+H].
Embodiment 3
[0037] Example 3: 2-(3-methyl-naphthalen-2-yl)-5-(3-methyl-4-(((1R, 3R, 5R, 7R)-2-methyladamantane-2- Synthesis of base)oxy)phenyl)pyridine
[0038]
[0039] 3-Methyl-naphthalene-2-boronic acid (0.375mmol), compound 4 (137.97mg, 0.375mmol), Na 2 CO 3 (119.8mg, 1.13mmol), DME (0.61mL) and H 2 O (0.15 mL) was added to a 5 mL microwave vial. vial with N 2 Degas for 11 minutes, then add PdCl 2 (dppf)CH 2 Cl 2 (33.1 mg, 0.045 mmol) adduct. The reaction mixture was heated at 120 °C for 50 minutes by microwave irradiation. The resulting mixture was diluted with ethyl acetate and filtered through celite, then concentrated in vacuo. Purification by flash chromatography using 0-100% ethyl acetate / heptane as eluent gave 2-(3-methyl-naphthalen-2-yl)-5-(3-methyl- 4-(((1R,3R,5R,7R)-2-methyladamantan-2-yl)oxy)phenyl)pyridine, 134.8 mg, 76% yield. LC-MS (ESI, pos, ion) m / z: 474[M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com