Tetrandrine derivatives and preparation method and application thereof in preparation of antitumor medicine
A technology of tetrandrine and its derivatives, which is applied in the field of preparation of tetrandrine derivatives and their application in the preparation of anti-tumor drugs, which can solve the problems of low cure rate and achieve simple preparation methods, inhibition of proliferation, and strong activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
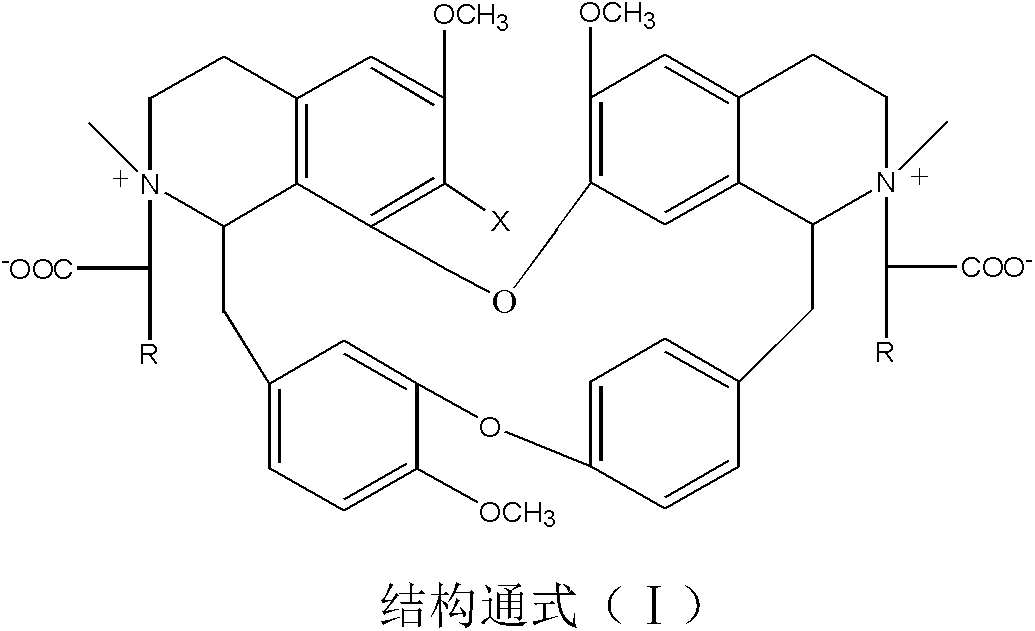
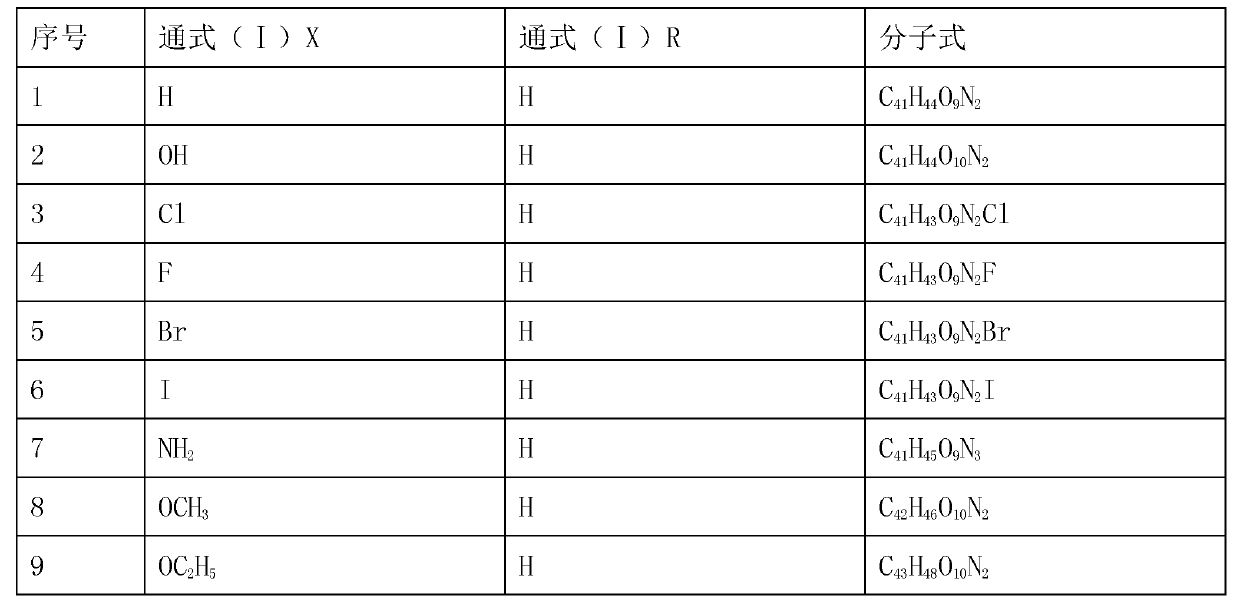
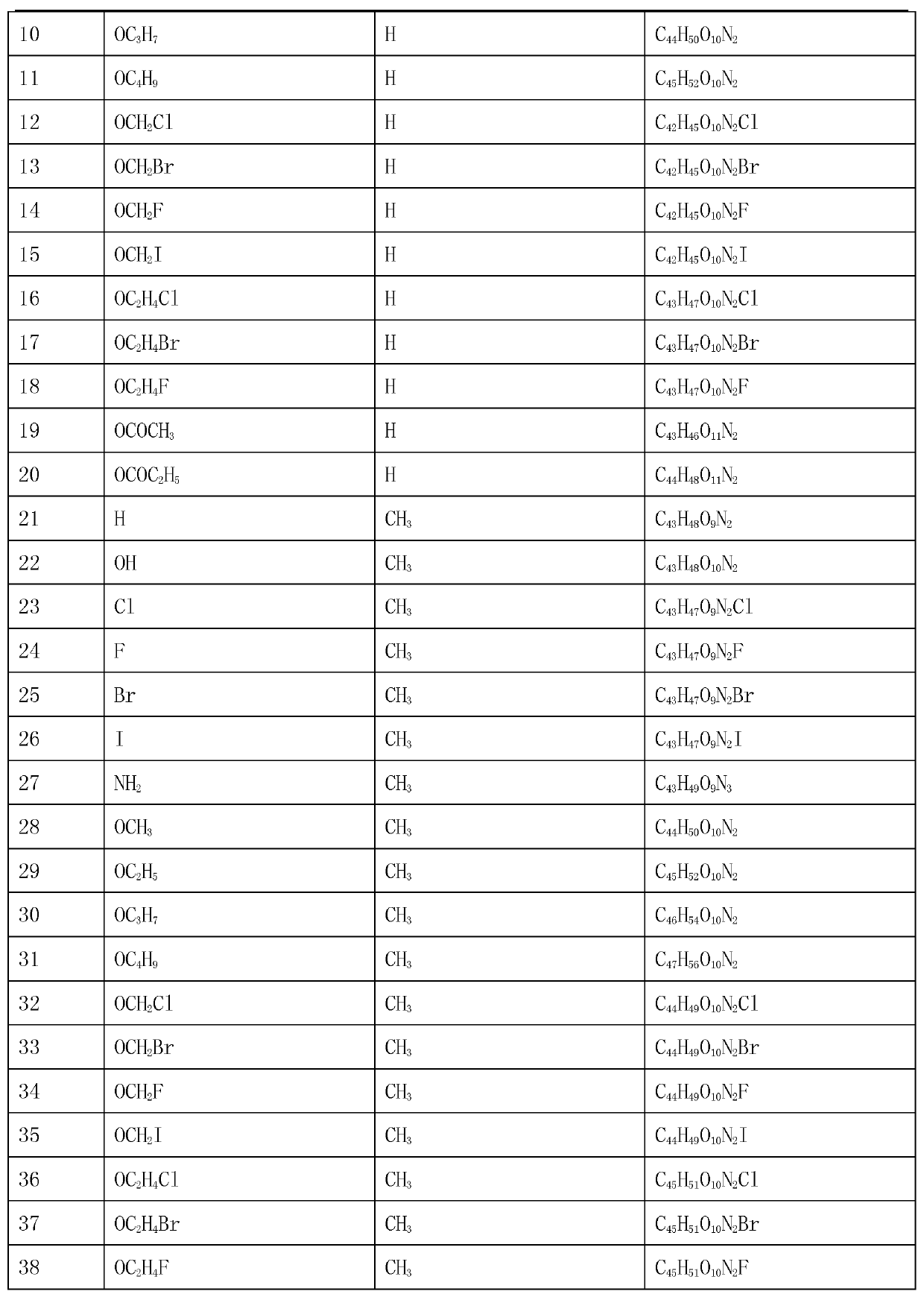
[0036] Weigh 6.06g of bisbenzylisoquinoline (in general formula II, X=H), 12.50g of sodium 2-chloroacetate and 0.40g of sodium hydroxide, add them to a 500mL three-necked flask, dissolve in 100mL propanol, heat and stir, The reaction was stirred at 83°C for 8 hours, the solvent was evaporated under reduced pressure, the temperature was reduced to room temperature, 10% HCl was neutralized to neutrality, 50 mL of water was added and the mixture was extracted 3 times with ethyl acetate (50 mL×3), and the extract was used anhydrous Na 2 SO 4 Drying for 8 hours, recovering ethyl acetate, TLC tracking the reaction and separation and purification of the product, the solid was dried at 60°C for 2 hours to obtain 6.21 g of light brown powder with a yield of 85.69% and a purity of 98.55% (HPLC). The melting point of the target product: 178-179℃, the time-of-flight mass spectrum: M / e(355.1601), the molecular formula is C 41 H 44 O 9 N 2 , 13 C NMR(75MHz, DMSO-d 6 ): δ20.63(C-4),23.22(C-4’),...
Embodiment 2
[0038] Weigh 6.22g of 7-hydroxybisbenzylisoquinoline (X=OH in general formula II), 10.00g of sodium 2-bromoacetate and 0.60g of potassium hydroxide, dissolve them in 100mL of n-butanol, add them to a 500mL three-necked flask , Heating and stirring to 118℃, and stirring at constant temperature for 6h, distilling off the solvent under reduced pressure, cooling to room temperature, 3%H 2 SO 4 Neutralize to neutral, add 50 mL of water and extract 3 times with chloroform (60 mL×3), and use anhydrous Na for the extract 2 SO 4 After drying for 8 hours, recovering chloroform, TLC followed the reaction and the separation and purification of the product, the solid was dried at 60°C for 2 hours to obtain 6.52 g of light red powder with a yield of 88.03% and a purity of 97.36% (HPLC). The melting point of the target product: 181-182℃, the time of flight mass spectrum: M / e(363.1575), the molecular formula is C 41 H 44 O 10 N 2 , 13 According to CNMR characterization, the main difference from ...
Embodiment 3
[0040] Weigh 6.40g of 7-chlorobisbenzylisoquinoline (X=Cl in the general formula II), 5.50g of sodium 2-iodoacetate, and γ-Al 2 O 3 -Na alkali 2.60g, add to a 500mL three-necked flask, add 200mL of water and stir and mix, cool to 10℃, react at a constant temperature for 48h, warm to room temperature and then filter, the filtrate is neutralized with dilute ammonia water, and the water is evaporated under reduced pressure to liquid volume Approximately 40 mL, crystallized overnight at 5°C, filtered, followed by TLC to track the reaction and separation and purification of the product. The obtained solid was dried at 60°C for 4h to obtain 3.63g of light red powder with a yield of 47.85% and purity. 98.15% (HPLC). The melting point of the target product: 176-178℃, the time of flight mass spectrum: M / e(372.1406), the molecular formula is C 41 H 43 O 9 N 2 Cl, 13 CNMR characterization, the main difference from Example 1 is 105.45 (C-7), which is compound 3 in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com