Quick in-vivo screening method of antagonistic trichoderma
A screening method, Trichoderma technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, fungi, etc., can solve the problems of slow re-screening speed of antagonistic Trichoderma, low efficiency of re-screening of antagonistic Trichoderma, and achieve saving materials, solve the heavy workload, and improve the effect of screening efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
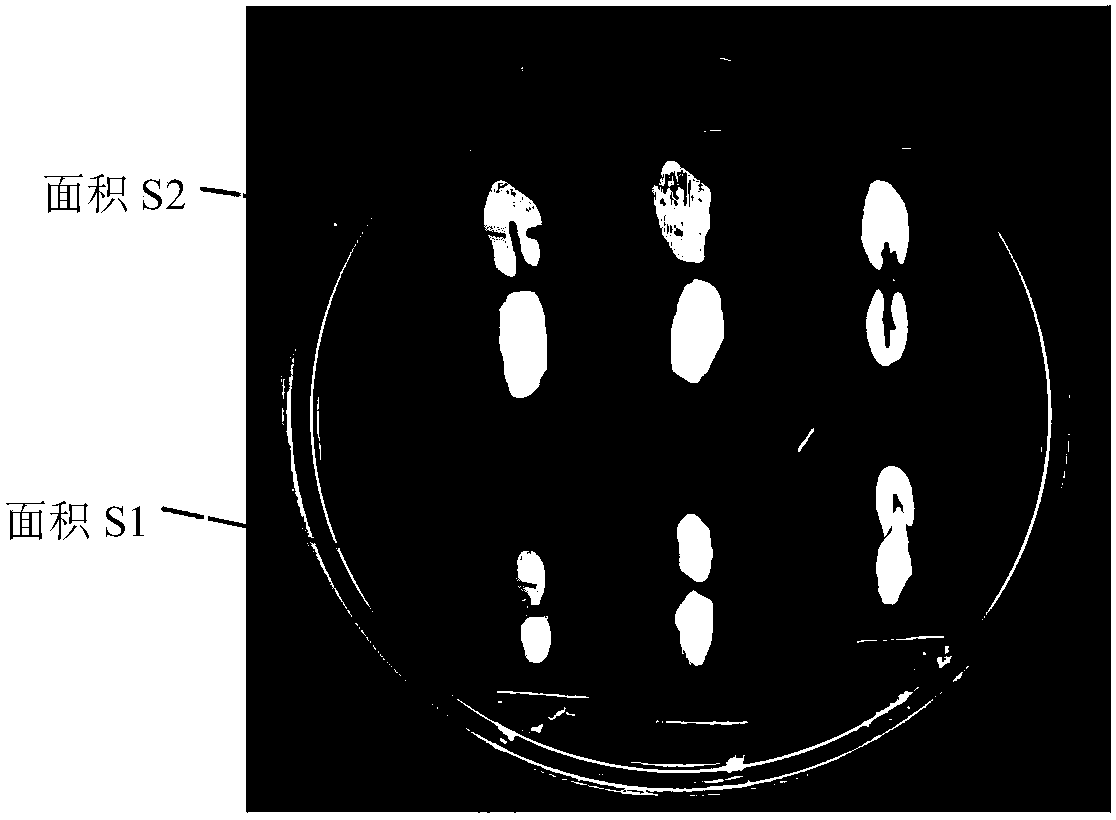
[0056] This embodiment relates to a rapid screening method for antagonizing Trichoderma living organisms, comprising the following steps:
[0057]1. Cultivate cucumber anthracnose bacteria (gifted by Li Baoju, a researcher at the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences) on the PDA plate for 7 days. Take the fungus cake from the edge of the colony, transfer it to PD liquid medium, and cultivate it in a constant temperature shaker at 28°C and 180r / min for 5 days. Transfer the cultured fermentation broth to a centrifuge tube, and centrifuge at 10000rr for 10min in a centrifuge to obtain the germ fermentation supernatant.
[0058] 2. In a 5ml centrifuge tube, mix the supernatant of the bacterial fermentation broth with MS medium at a ratio of 1:4, with a total volume of 4ml, to obtain the bacterial inoculum.
[0059] 3. Put the cucumber seeds (Xiafeng No. 2) in a petri dish covered with moist filter paper, and germinate them in a constant temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com