Pig mycoplasma pneumonia inactivated vaccine and preparation method thereof
A technology of inactivated vaccine, Mycoplasma hyopneumoniae, which is applied in the field of inactivated vaccine against Mycoplasma hyopneumoniae and its preparation, can solve the problems of difficult vaccine development and difficult estimation of vaccine protection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1——vaccine preparation
[0074] 1. Seed Preparation for Production
[0075] After unsealing the freeze-dried strains, dissolve them in the culture medium, inoculate the modified Goodwin’s A26 liquid medium, and culture at 37°C for 3 to 7 days, until the color of the culture medium turns yellow, becomes slightly turbid, and the pH of the bacterial solution drops to 6.8 At about 10% of the inoculum size, continue for 1 generation, culture at 37°C for 3 to 7 days, and harvest when the color of the medium turns yellow, becomes slightly turbid, and the pH value of the bacterial solution drops to about 6.8. After passing the pure inspection, as a primary seed. Take the first-grade seed bacteria solution and inoculate it in the improved Goodwin's A26 liquid medium according to the inoculum amount of 10%. Shake culture at 37°C for 3 to 7 days, and harvest when the color of the medium turns yellow, slightly turbid, and the pH value of the bacterial solution drops to...
Embodiment 2
[0094] Embodiment 2 - the safety test of vaccine
[0095] Three batches of Mycoplasma hyopneumoniae inactivated vaccines were prepared, which were inactivated by BEI and then concentrated. Montanide GEL01RP water-soluble adjuvant was added to make three batches of inactivated vaccines against Mycoplasma hyopneumoniae. The batch numbers were 001, 002 and 003, respectively. The product has been tested for safety. Prepare 3 batches of vaccines, single-dose inoculation to the minimum use age piglets, single-dose re-inoculation to piglets, single-dose over-dose inoculation to piglets, single-dose re-inoculation to pregnant animals, over-dose re-inoculation to pregnant animals, The safety of single-dose inoculation of animals and over-dose inoculation of breeding boars was tested. The results showed that: during the observation period, the spirit and food intake of the experimental animals were normal, and the body temperature after inoculation was normal without significant changes...
Embodiment 3
[0096] Embodiment 3——The immunity test of vaccine
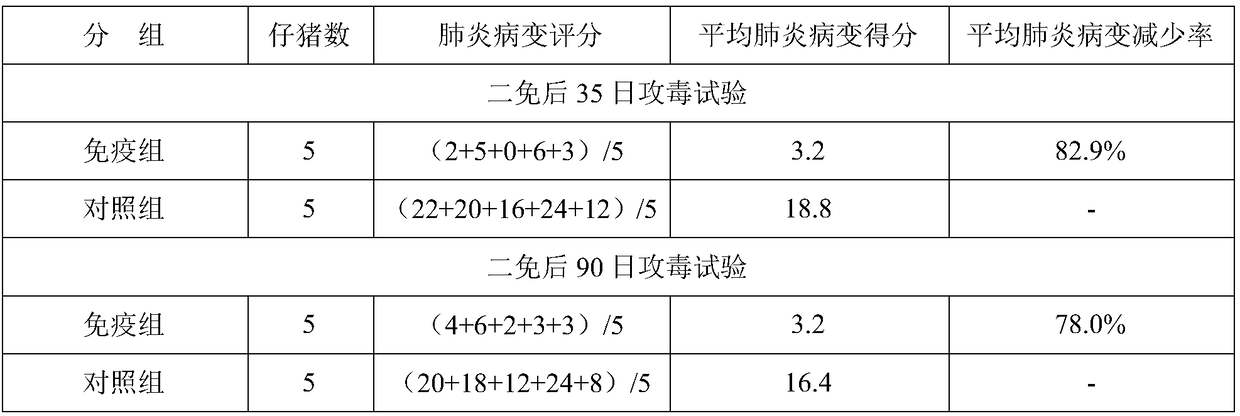
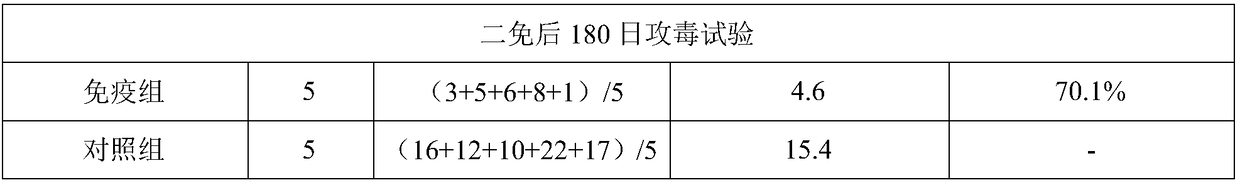
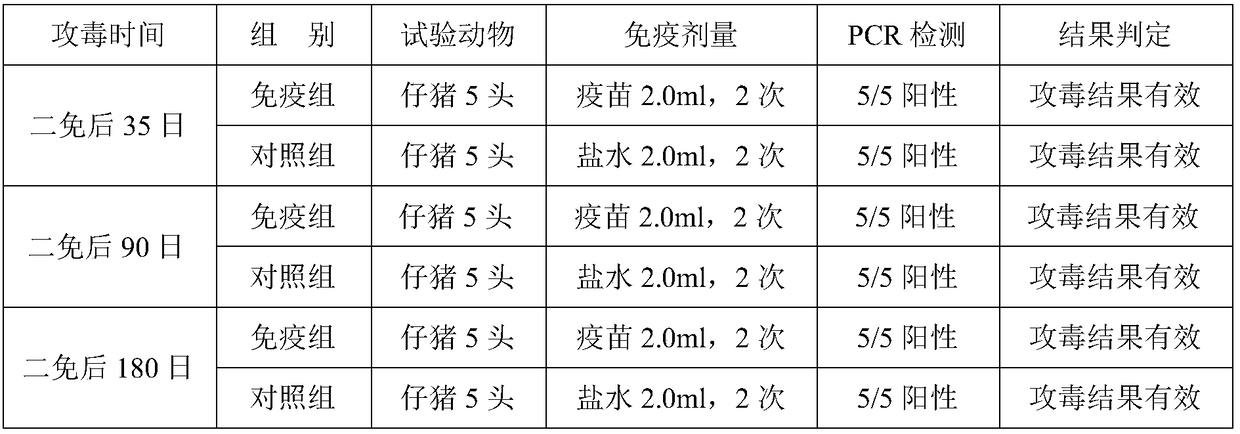
[0097] The following immune efficacy tests were carried out with the prepared 3 batches of vaccines: the immune efficacy test of the inactivated mycoplasma pneumoniae vaccine on the target animal piglets; the immune efficacy test of the inactivated mycoplasma pneumoniae vaccine on the target animal pregnant sows. The test results are as follows:
[0098] (1) The immune efficacy test of the inactivated Mycoplasma pneumoniae vaccine on piglets aged 2 to 3 weeks, the piglets aged 2 to 3 weeks were inoculated intramuscularly with the inactivated Mycoplasma pneumoniae vaccine, 2.0ml / head, followed by the same dosage and route 21 days later. Strengthen the immunization, 35 days after the second immunization, the immunization group and the control group were intratracheally injected with 5.0ml of virulent Mycoplasma hyopneumoniae virulent ZY strain-containing diseased lung tissue suspension to challenge the virus, and observed for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com