Drug comprising aripiprazole and cilostazol
A technology of aripiprazole and cilostazol, applied in the field of combination of aripiprazole and cilostazol, can solve problems such as mental changes, showing mental changes and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0160] 1.1: In vivo test (medium cerebral artery occlusion model)
[0161] In this experiment, 30 male 10-week-old C57BL / 6J mice were divided into the following groups. To each group, 6 mice were assigned.
[0162] Grouping
[0163] 1) Control group
[0164] 2) Vehicle giving group
[0165] 3) Cilostazol administration group alone
[0166] 4) Aripiprazole alone administration group
[0167] 5) Cilostazol and Aripiprazole administration group
[0168] A 20% DMSO aqueous solution was used as the vehicle. The dose of cilostazol and aripiprazole were 3 mg / kg each. For the cilostazol and aripiprazole groups, the dose of cilostazol and aripiprazole were also 3 mg / kg each. Each drug was dissolved in 20% DMSO aqueous solution and administered to mice.
[0169] Test schedule
[0170] All mice were given a 14-day adaptation period. On the day after the last day of the adaptation period, 4 groups of mice other than the control group were subjected to the operation described later to induce midd...
example 2
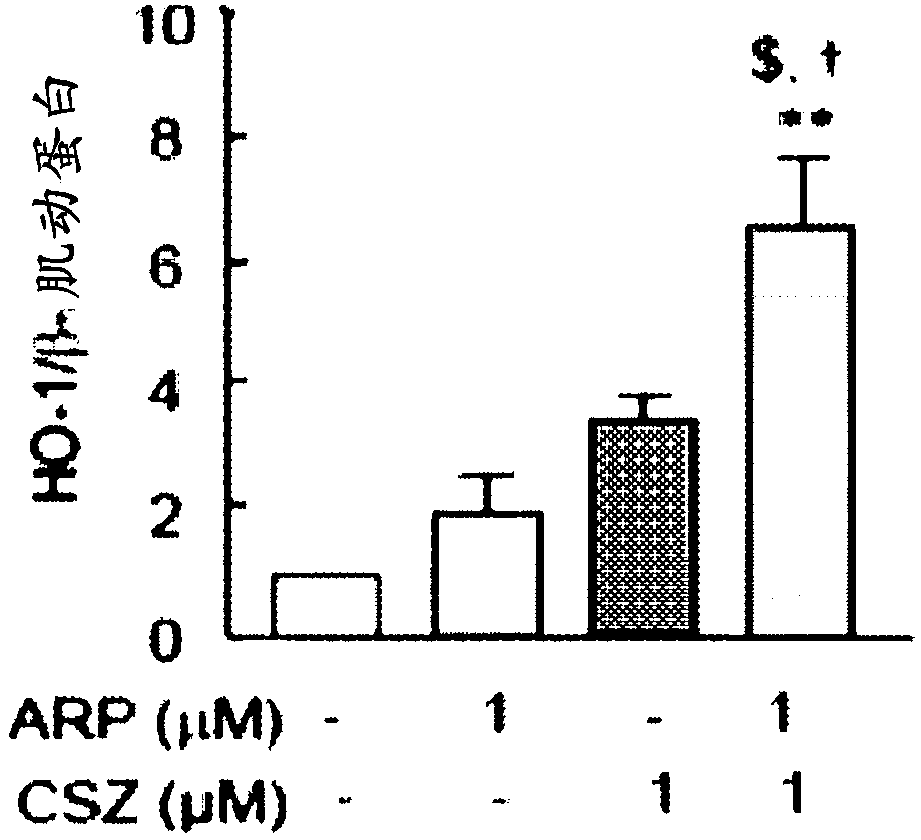
[0206] 2.1: In vitro testing
[0207] To evaluate the effects of aripiprazole and / or cilostazol on neuronal cells. Specifically, the expression level of heme oxygenase-1 ("HO-1") was measured using Western blotting. HO-1 is a neuroprotective protein that protects cells from damage caused by oxidative stress.
[0208] HT22 neuronal cells (a neuronal cell line derived from the mouse hippocampus) in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 units / mL penicillin, and 100 μg / mL streptomycin (DMEM). Thereafter, the cultured cells were divided into the following 4 groups: [1] untreated group, [2] group treated with aripiprazole (1 μM), [3] group treated with cilostazol (1 μM), and [ 4] A group treated with aripiprazole (1 μM) + cilostazol (1 μM), and the separated cells were treated separately. Each treatment was performed by exposing the cell group to each substance for 6 hours. After that, the treated cells were collected and lysed in a lysis buffer ...
example 3
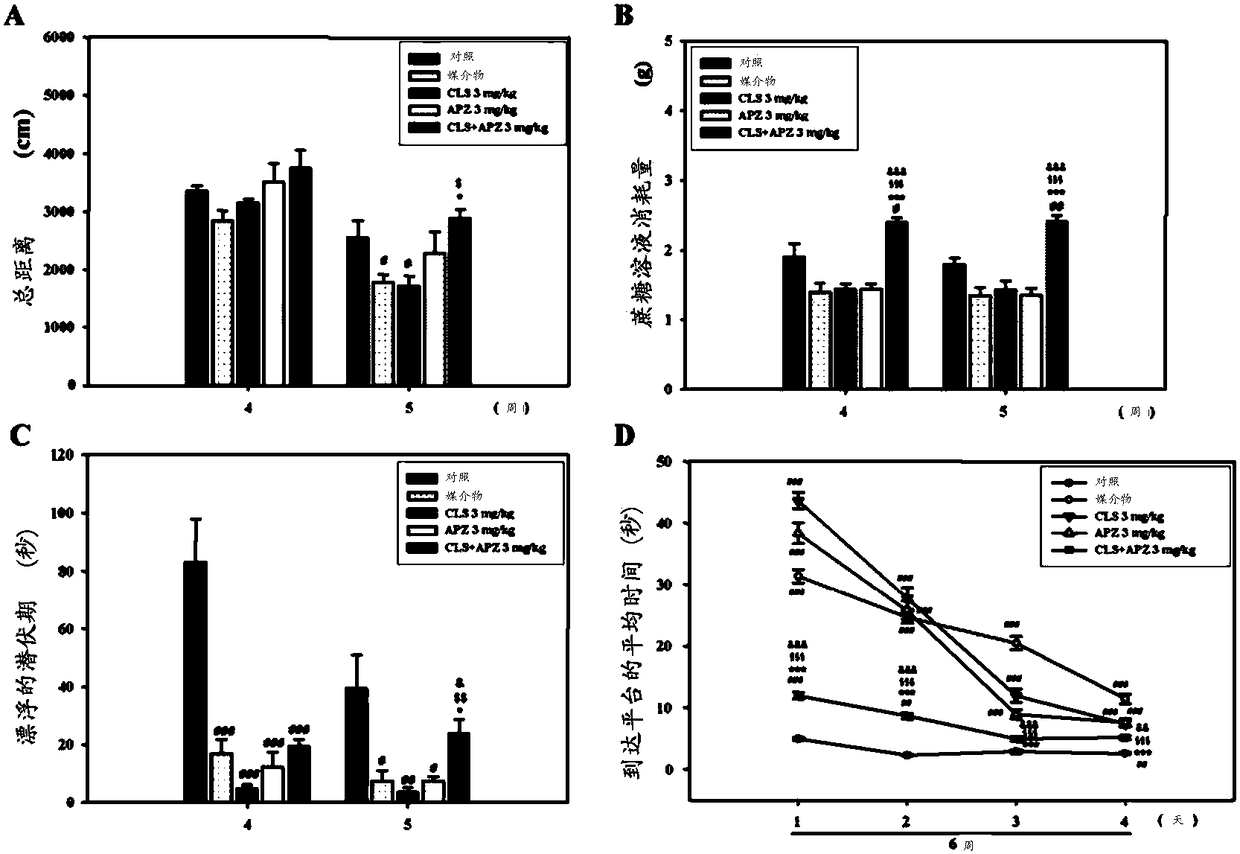
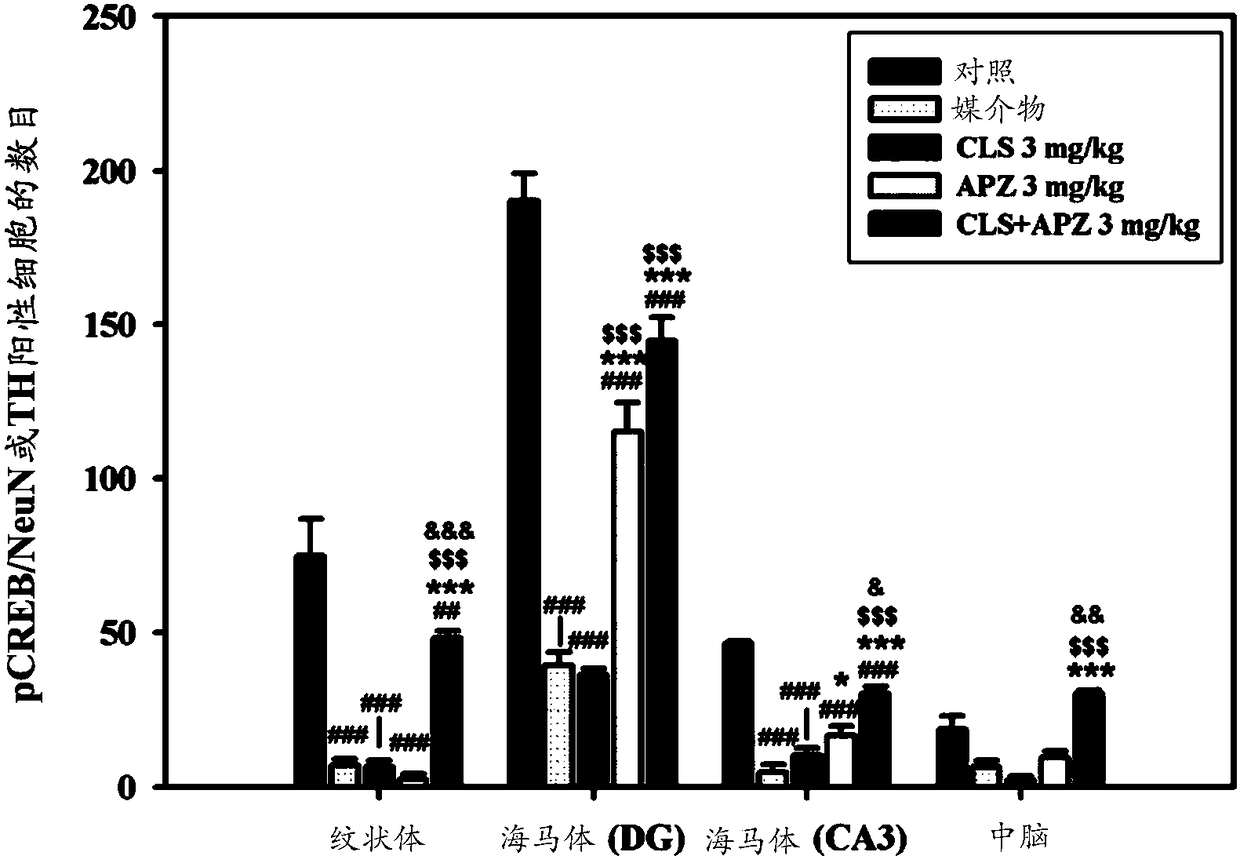
[0215] 3.1: In vivo test (bilateral carotid artery stenosis model)
[0216] A mouse model with bilateral common carotid artery stenosis was prepared, and the Morris water maze test was performed on the mouse model to evaluate spatial learning and memory capabilities. Forty male 10-week-old C57BL / 6J mice (purchased from Koatech, Seoul, Korea) were divided into the following groups and used. To each group, 8 mice were assigned.
[0217] Grouping
[0218] 1) Control group
[0219] 2) Vehicle giving group
[0220] 3) Aripiprazole alone administration group
[0221] 4) Cilostazol alone administration group
[0222] 5) Cilostazol and Aripiprazole administration group
[0223] A 25% DMSO aqueous solution was used as the vehicle. Also for the cilostazol and aripiprazole administration groups, the dose of aripiprazole is 0.5 mg / kg, and the dose of cilostazol is 20 mg / kg. Each drug was dissolved in 25% DMSO aqueous solution and orally administered to mice.
[0224] Test schedule
[0225] Give a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com