Low cost oxidation catalysts for VOC and halogenated VOC emission control
An oxidation catalyst, tin oxide technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, catalyst activation/preparation, etc. and low-cost application improvements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
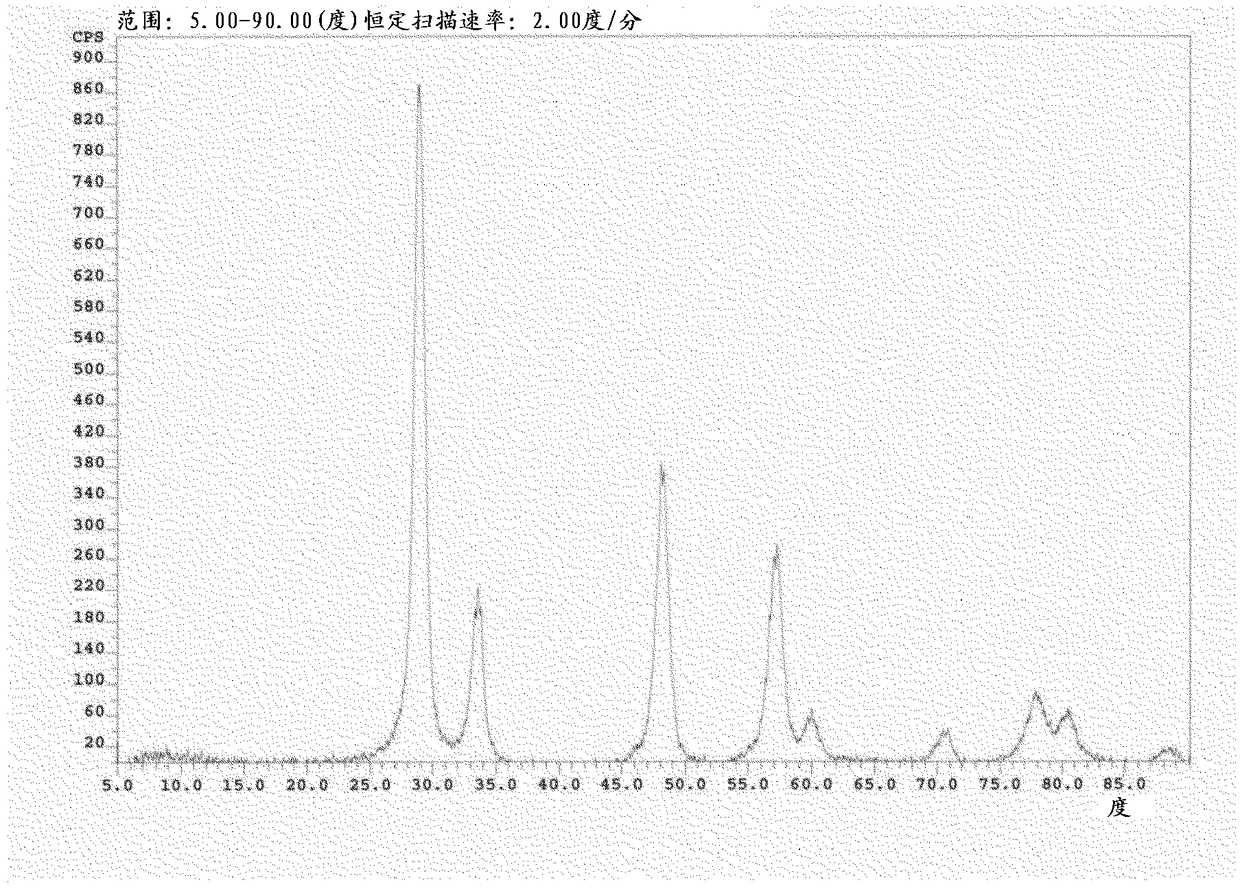
[0035] Through the solid solution of ceria-zirconia powder (see figure 1 ) and silica in a 5:1 ratio with sufficient deionized water to prepare a washcoat slurry. Ceria-zirconia powder has about 85m 2 / g surface area. Once mixed, the slurry is ground to the desired particle size. A ceramic monolithic substrate having a pore density of 400 cpsi was partially dipped into the washcoat slurry. Excess slurry was removed using an air knife. The coated part was dried at about 150°C and calcined at about 500°C to give a part with a washcoat loading of about 170 g / L. On the coated part, a ruthenium nitrate solution was deposited by wet dipping, dried at 150°C and calcined at 500°C to achieve a ruthenium loading of about 2.8 g / L measured by known techniques.
Embodiment 2
[0037] Use silica-stabilized titanium dioxide powder (with about 85m 2 surface area per gram), colloidal silica, and a sufficient amount of deionized water, and then grind the mixture to the desired particle size to prepare a washcoat slurry. A 400 cpsi monolithic substrate was then partially dipped into the washcoat slurry. Excess slurry was removed using an air knife. The coated part was dried at 150°C and calcined at 500°C. The resulting fraction had a washcoat loading of about 180 g / L. On the coated part, a ruthenium nitrate solution was deposited by wet dipping, dried at 150°C and calcined at 500°C to achieve a ruthenium loading of about 2.6 g / L.
Embodiment 3
[0039] Use silicon dioxide stabilized tin oxide powder (with about 110m 2 surface area per gram), colloidal silica, and a sufficient amount of water, and then grind the mixture to the desired particle size to prepare a washcoat slurry. A 400 cpsi monolithic substrate was partially dipped into the washcoat slurry. Remove excess slurry using vacuum suction or air knife. The coated part was dried at about 150°C and calcined at 500°C. This yielded a fraction with a washcoat loading of about 180 g / L. On the coated part, a ruthenium nitrate solution was deposited by wet dipping or other suitable technique, dried at 150°C and calcined at about 500°C to achieve a ruthenium loading of about 2.6 g / L.
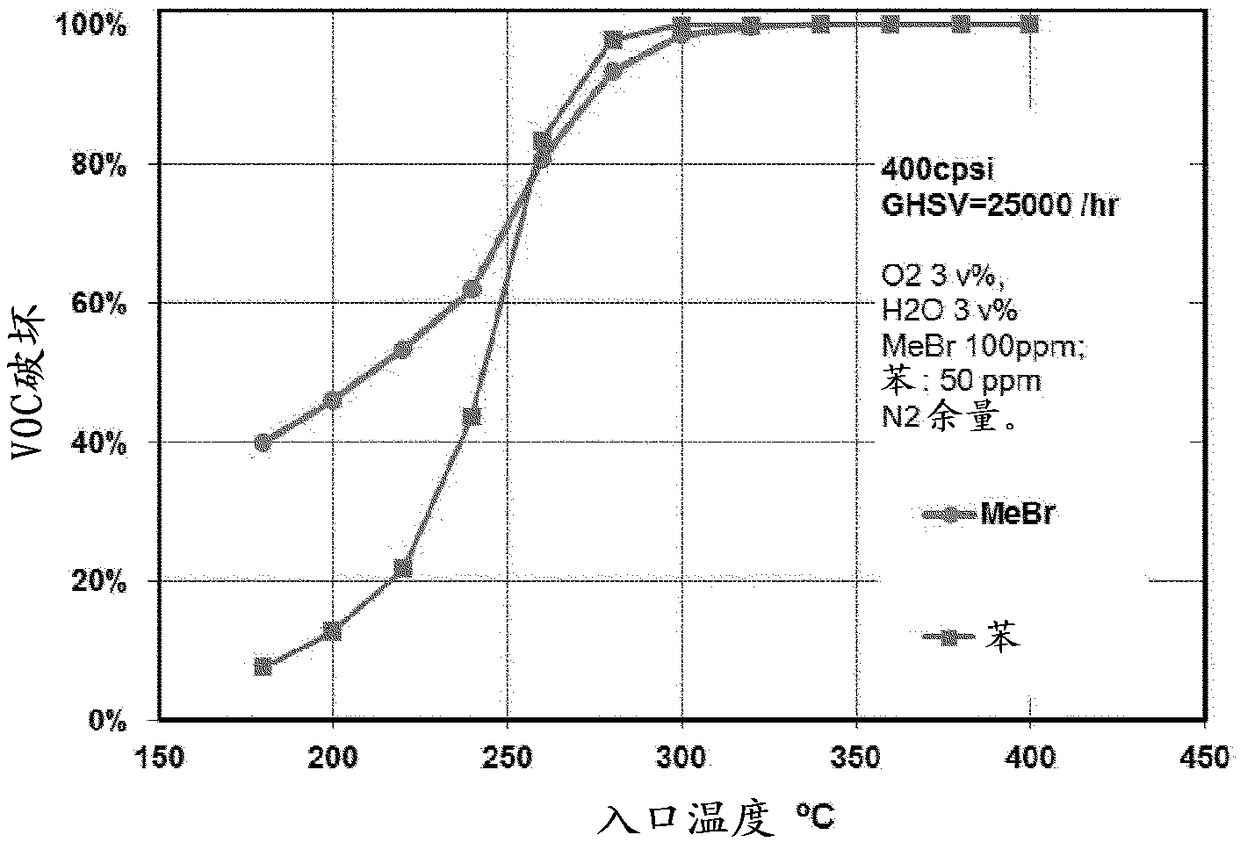
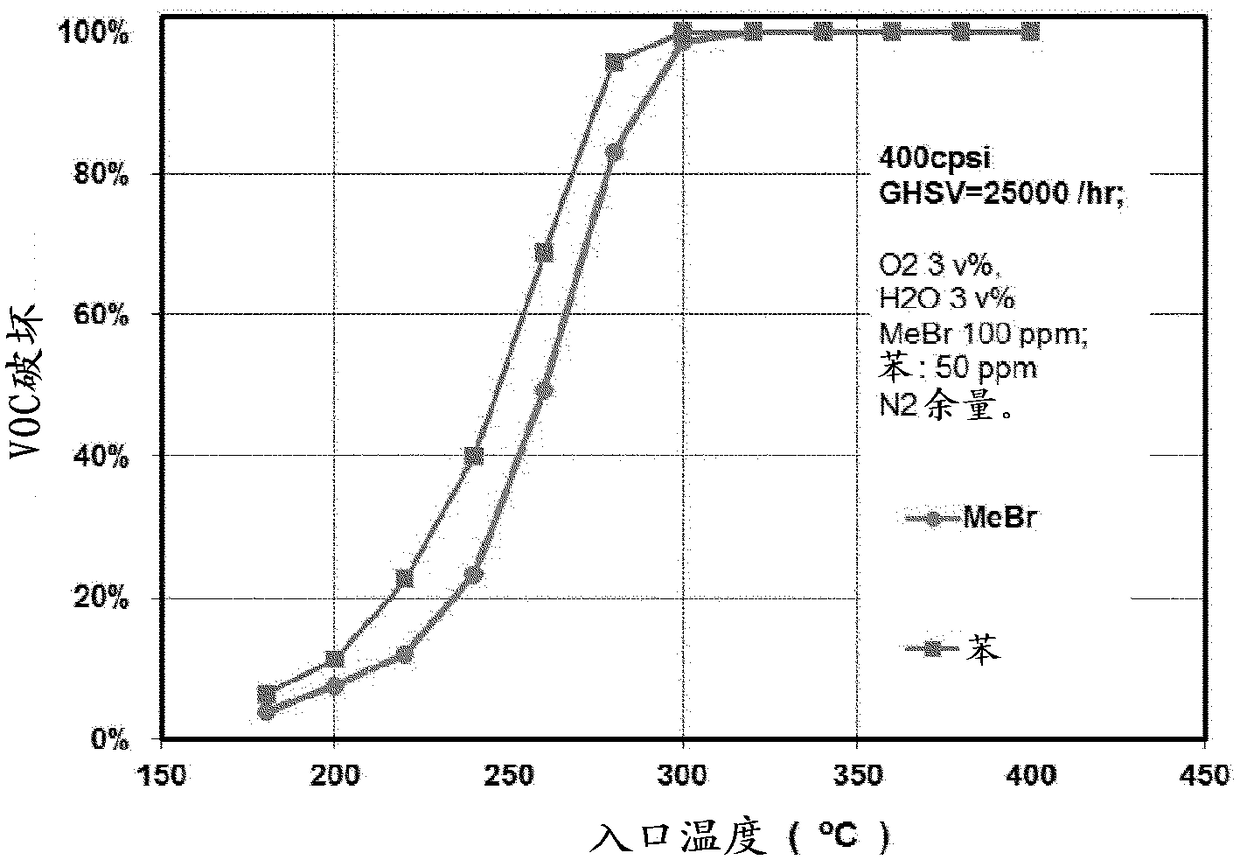
[0040] The catalysts described in the above examples were evaluated for their catalytic activity. The reactivity was tested on a laboratory scale reactor based on core samples of the catalyst cut from the catalyst monolith sections of each of the above examples. The hazardous compoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com