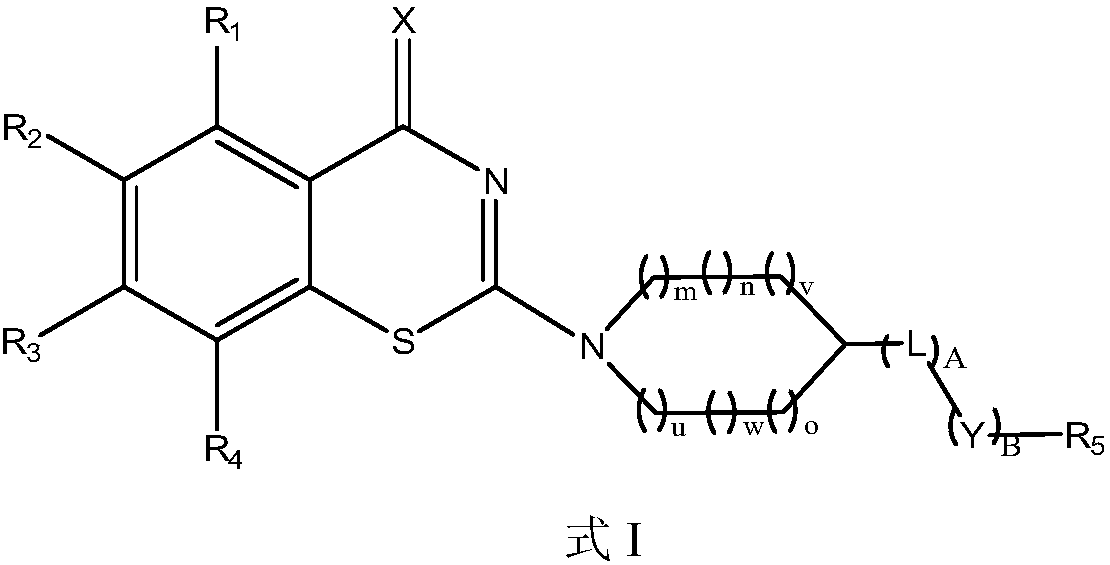
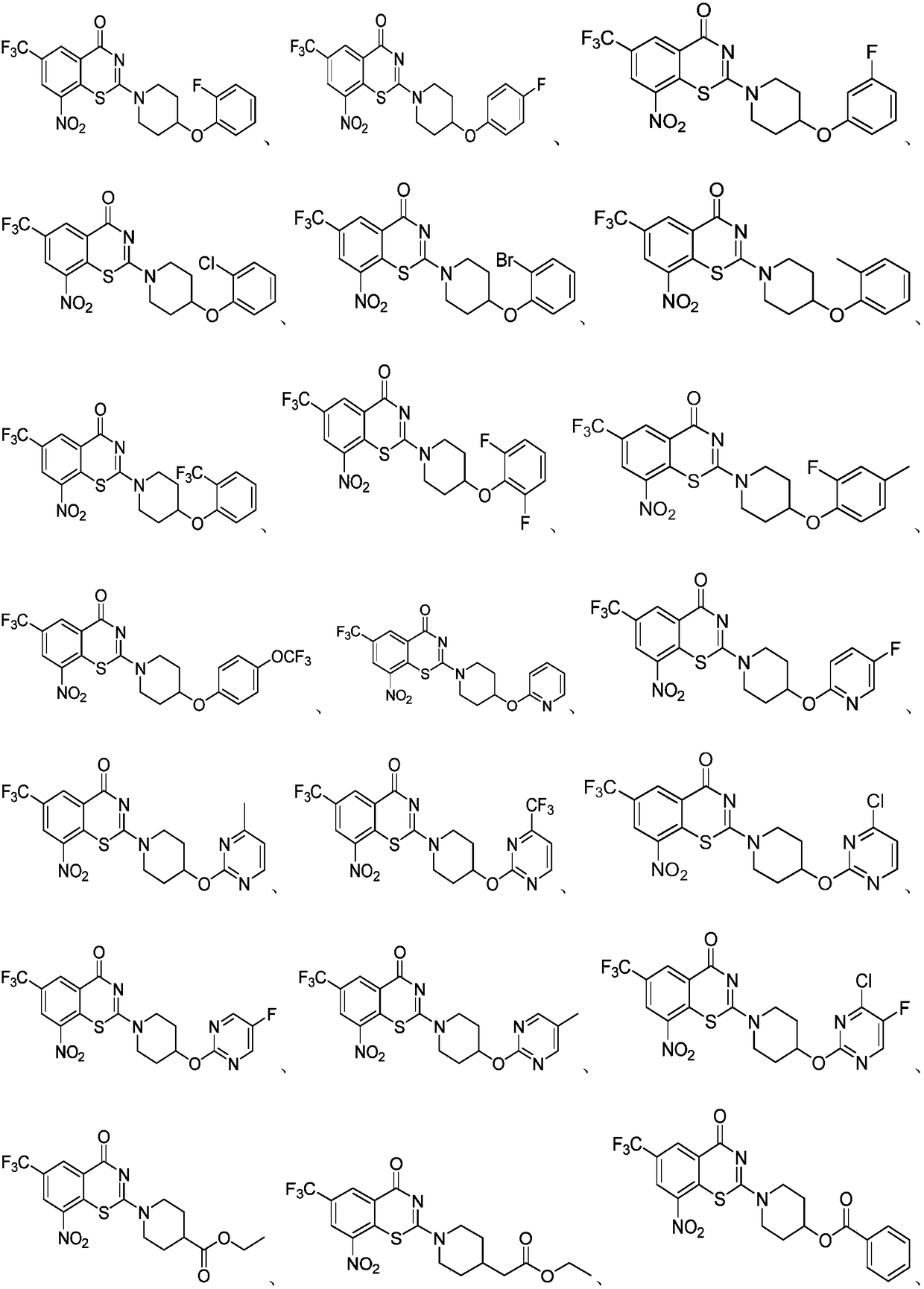
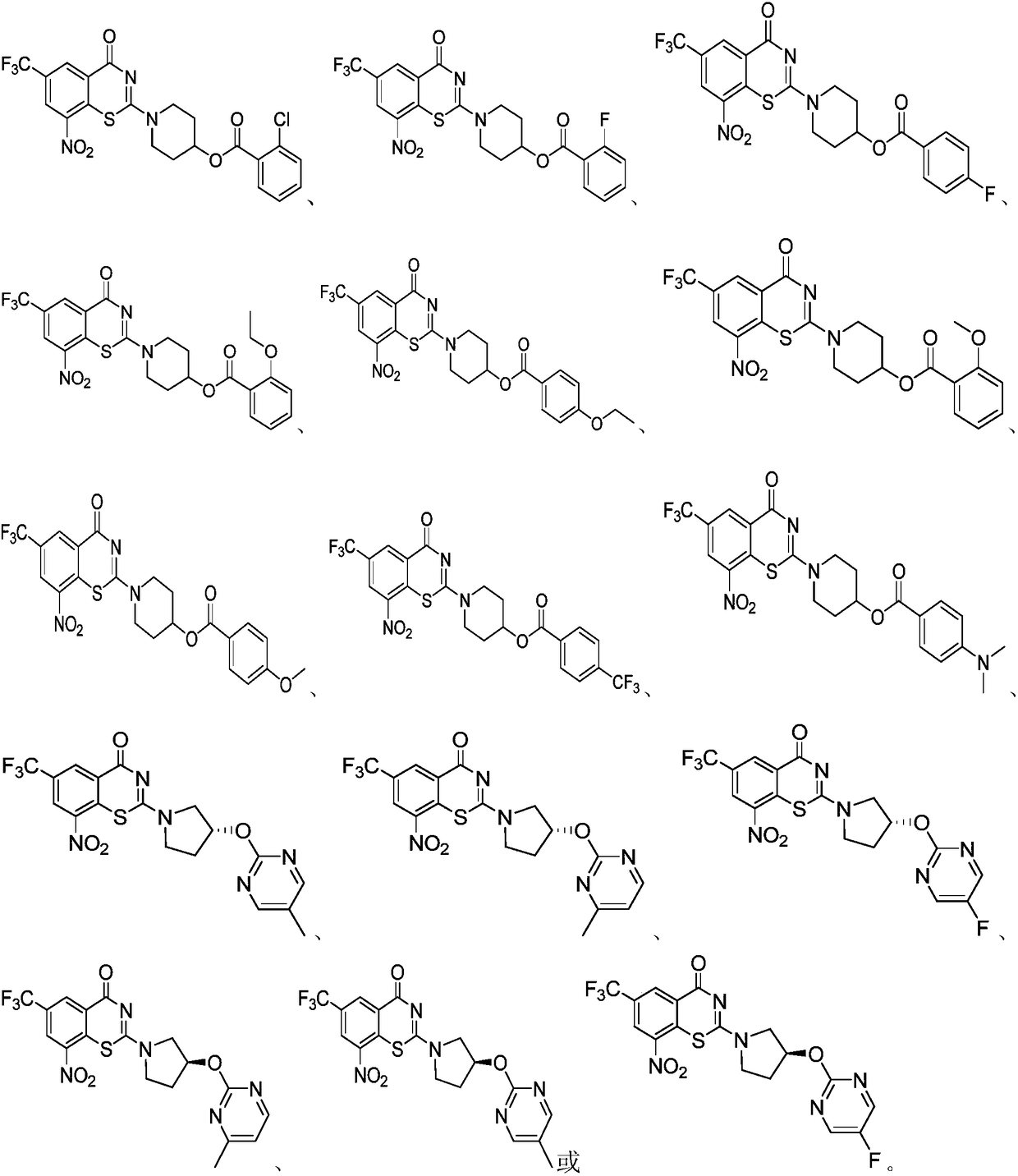
Benzothiazine derivative, a preparation method and uses thereof
A technology of benzothiazine and derivatives, which is applied in the field of benzothiazine derivatives and its preparation, and can solve problems such as inability to guarantee compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Compound 1a 2-(4-(2-fluorophenoxy)piperidin-1-yl)-8-nitro-6-(trifluoromethyl)-4H-benzo[e][1, 3] Preparation of Thiazin-4-one
[0098]
[0099] 1.1. Dissolve N-Boc-4-hydroxypiperidine, 2-fluorophenol, and triphenylphosphine in tetrahydrofuran, add diethyl azodicarboxylate dropwise to the above solution, and stir at room temperature for reaction. The end of the reaction was monitored by TLC, concentrated to dryness, and purified by silica gel column chromatography to obtain intermediate 2. Intermediate 2 was dissolved in dichloromethane, trifluoroacetic acid was added dropwise, and the reaction was stirred at room temperature. TLC monitored the completion of the reaction and concentrated to dryness. Diluted with dichloromethane, washed with saturated sodium bicarbonate solution, washed the organic phase with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain intermediate 3 (4-(2-fluorophenoxy)piperidine).
[0100] 1....
Embodiment 2
[0103] Example 2 Compound 1b 2-(4-(4-fluorophenoxy)piperidin-1-yl)-8-nitro-6-(trifluoromethyl)-4H-benzo[e][1, 3] Preparation of Thiazin-4-one:
[0104]
[0105] 2.1. The preparation method of 4-(4-fluorophenoxy)piperidine is the same as 1.1, and the raw materials are N-Boc-4-hydroxypiperidine and 4-fluorophenol.
[0106] 2.2. Add 20 mL of dichloromethane to 2-chloro-3-nitro-5-trifluoromethylbenzoic acid (12 mmol), slowly add oxalyl chloride (30.5 mmol) and 0.05 mL of DMF dropwise under stirring at room temperature, and react for two hours . After the reaction, the reaction solution was spin-dried, dissolved in 15 mL of dichloromethane, slowly added dropwise to ammonium thiocyanate (36 mmol), then added PEG-400 (0.2 g), stirred at room temperature for 1.5 hours; filtered, and the filtrate was added dropwise to 4- (4-fluorophenoxy)piperidine in dichloromethane solution, stirred at room temperature for 40 minutes. After the reaction was completed, the reaction solution was ...
Embodiment 3
[0109] Example 3 Compound 1c 2-(4-(3-fluorophenoxy)piperidin-1-yl)-8-nitro-6-(trifluoromethyl)-4H-benzo[e][1, 3] Preparation of Thiazin-4-one:
[0110]
[0111] 3.1. The preparation method of 3-(3-fluorophenoxy)piperidine is the same as 1.1, and the raw materials are N-BBoc-4-hydroxypiperidine and 3-fluorophenol;
[0112] 3.2. Add 20 mL of dichloromethane to 2-chloro-3-nitro-5-trifluoromethylbenzoic acid (12 mmol), slowly add oxalyl chloride (30.5 mmol) and 0.05 mL of DMF dropwise under stirring at room temperature, and react for two hours . After the reaction, the reaction solution was spin-dried, dissolved in 15 mL of dichloromethane, slowly added dropwise to ammonium thiocyanate (36 mmol), then added PEG-400 (0.2 g), stirred at room temperature for 1.5 hours; filtered, and the filtrate was added dropwise to 3- (3-fluorophenoxy)piperidine in dichloromethane solution, stirred at room temperature for 40 minutes. After the completion of the reaction, the reaction solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com