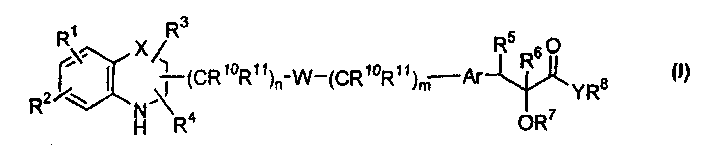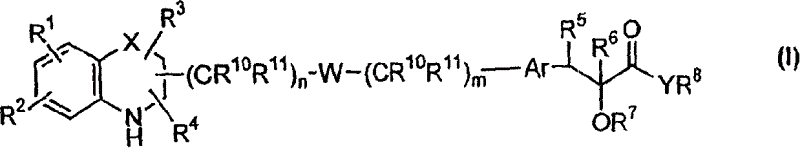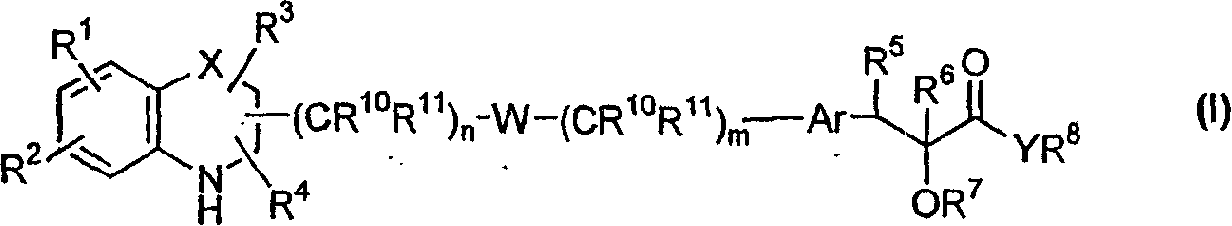Benzoxazine and benzothiazine derivatives and parmaceutical compositions containing them
A derivative and benzo-based technology, applied in the field of benzoxazine and benzothiazine derivatives and pharmaceutical compositions containing them, can solve problems such as high CVD risk, and achieve the effect of reducing total cholesterol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0230] According to another embodiment of the present invention, the compound of general formula (I)——Y represents oxygen; W represents -O-aryl-(CR 10 R 11 )o-NR 12 -; all other symbols as defined above - may be prepared by means of a process comprising:
[0231] Use a solvent such as CH 2 Cl 2 、CHCl 3 , chlorobenzene, benzene, THF, in the presence of catalysts such as p-toluenesulfonic acid, methanesulfonic acid, TFA, TfOH, BF 3 -OEt 2 etc., make formula (IIIi) compound
[0232]
[0233] where n and p are integers 0-6, G 1 for NH 2 or formyl, all other symbols are as defined above,
[0234] Reaction with a compound of formula (IIIh),
[0235]
[0236] where q is an integer 0-6, G 2 for NH 2 or formyl, all other symbols are as defined above. The reaction can also be performed using activated molecular sieves. The reaction temperature may be in the range of 10°C to 100°C, preferably in the range of 10°C to 60°C. Na(CN)BH can be used in solvents such as meth...
preparation example 1
[0333] Ethyl 2-ethoxy-3-(4-aminophenyl)propionate
[0334]
[0335] step (i)
[0336]From triethyl 2-ethoxyphosphonoacetate (26.5g, 1.5eq, 99.3mmol) and NaH (50% oil) (5.3g, 2eq, 132.4mmol) in THF (350ml) at 0°C Preparation of Wittig salts. To this was added solid 4-nitrobenzaldehyde (10 g, 1 eq, 66.2 mmol) in portions at 0° C., and the resulting solution was stirred at RT for 16 hours. The reaction mixture was diluted with ethyl acetate and washed with aqueous NH 4 Cl wash. The crude product contained the Z and E stereoisomers of ethyl 4-nitro-2-ethoxycinnamate (11 g).
[0337] step (ii)
[0338] At RT, in ethyl acetate (150ml), ethyl 4-nitro-2-ethoxycinnamate obtained in step (i) was washed with Pd (10%) / C-H 2 (60 psi) (11 g) was hydrogenated and purified by chromatography eluting with ethyl acetate / hexanes to afford the title compound as a viscous oil (9.41 g, 60% yield).
[0339] 1 H NMR (200MHz, CDCl 3 )δ: 1.16(t, J=7.0Hz, 3H), 1.22(t, J=7.0Hz, 3H), 2.90(d,
...
preparation example 2
[0345] 3-(3,4-Dihydro-2H-benzo[b][1,4]oxazin-4-yl)propyl bromide
[0346]
[0347] 3,4-Dihydro-2H-benzo[b][1,4]oxazine (3.0g, 1eq, 22.2mmol), 1,3-dibromopropane (22.5ml, 10eq, 222mmol) and anhydrous A mixture of sodium carbonate (7.05g, 3eq, 66.6mmol) in anhydrous DMF (200ml) was heated at 70°C for 16 hours. The reaction mixture was diluted with ethyl acetate, washed with water and brine. The residue was chromatographed eluting with ethyl acetate and hexanes to afford the title compound as a liquid (2.6 g, 47%).
[0348] 1 H NMR (200MHz, CDCl 3 )δ: 2.10-2.30(m, 2H), 3.37(t, J=4.4Hz, 2H), 3.40-3.56(m, 4H), 4.25(t, J=4.3Hz, 2H), 6.60-6.90(m , aromatic, 4H).
[0349] Mass m / z(CI): 255(M( 79 Br)), 256(M( 79 Br)+1), 257(M(Br 81 )), 258(M(Br 81 )+1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com