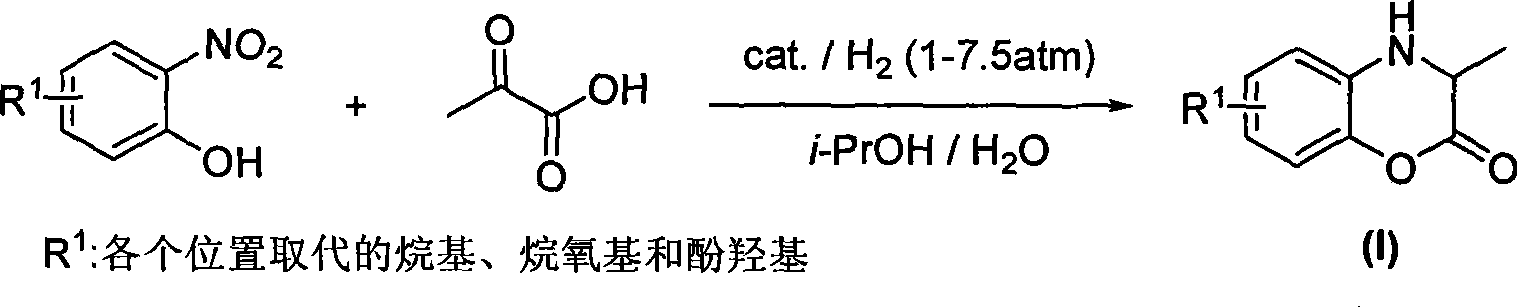
Method for preparing polysubstituted 3,4-dihydro-3-methyl-2H-1,4-benzoxazine-2-one
A multi-substituted, benzoxazine technology, applied in the field of preparing multi-substituted 3,4-dihydro-3-methyl-2H-1,4-benzoxazin-2-one, can solve the harsh reaction conditions , high cost of raw materials, cumbersome operation steps, etc., to achieve the effect of easy processing and reduced environmental protection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Grind coconut shell activated carbon and sieve to obtain activated carbon powder of 300-400 mesh. Add a certain amount of activated carbon to 10wt.% nitric acid, stir in 80°C water bath for 2 hours, then filter and wash with distilled water until neutral. Water vapor is then processed, dried, and set aside. Dissolve chloroplatinic acid in deionized water to make 1.7×10 -2 mol / L chloroplatinic acid solution. Add 10g of activated carbon treated with amine acid into 165mL of chloroplatinic acid aqueous solution, soak for 2 hours, then add 8mL of formaldehyde for reduction, and at the same time add saturated sodium bicarbonate solution dropwise, control the pH value to about 9, and react until the pH value remains unchanged. Filter and dry to obtain a Pt / C catalyst with a Pt content of 5 wt.%.
[0021] The reaction process is that 0.10mol o-nitrophenol, 0.12moL pyruvic acid, 2g 5wt.% Pt / C catalyst, 240mL 80v / v% isopropanol aqueous solution are added in a 0.5L autoclave wi...
Embodiment 2
[0023] Dissolve palladium chloride in deionized water to make a 1.7×10 -2 mol / L palladium chloride solution, get 278mL, add 10g coconut shell activated carbon (300 mesh) processed by the method of Example 1 and soak for 2h, then add 8ml formaldehyde for reduction, drop saturated sodium bicarbonate solution simultaneously, control the pH value is about 9, react until the pH value remains unchanged, filter and dry to obtain a Pd / C catalyst with a Pd content of 5wt.%.
[0024] Add 0.10mol of o-nitrophenol, 0.20mol of pyruvic acid, 2g of 5wt.% Pd / C catalyst, and 240mL of 80v / v% methanol aqueous solution into a 0.5L autoclave with a mechanical stirring device, and completely fill the air in the autoclave with hydrogen After the replacement, under the conditions of 2.0 MPa hydrogen pressure and 20° C., turn on the mechanical stirring at a speed of 1000 rpm, and react for 10 h. After the reaction was completed, the catalyst was directly filtered, 10 mL of concentrated hydrochloric a...
Embodiment 3
[0026] In the same manner as in Example 2, using the 5wt.% Pd / C catalyst that has been used 20 times, add 0.10mol o-nitrophenol, 0.20mol pyruvic acid, 240mL 80v / v% isopropyl Add the alcohol aqueous solution into a 0.5L autoclave with a mechanical stirring device, completely replace the air in the autoclave with hydrogen, and then turn on the mechanical stirring at a hydrogen pressure of 2.0MPa and 20°C at a speed of 1000rpm, and react for 10h . After the reaction was completed, the catalyst was directly filtered, 10 mL of concentrated hydrochloric acid was added to the filtrate, and the solvent was removed by distillation under reduced pressure using a rotary evaporator. 100 mL of dichloromethane and 300 mL of saturated aqueous sodium bicarbonate were added, and the solution was separated to obtain an oil phase and an aqueous phase. Separate the oil phase, add anhydrous magnesium sulfate to the oil phase to remove water, filter to obtain an anhydrous oil phase, and then conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com