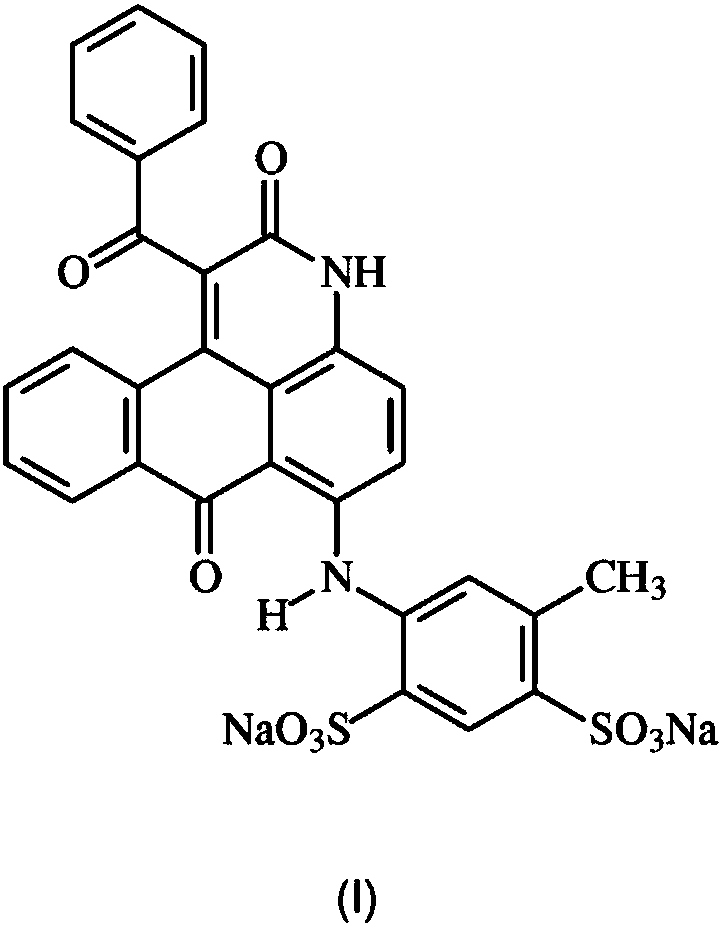
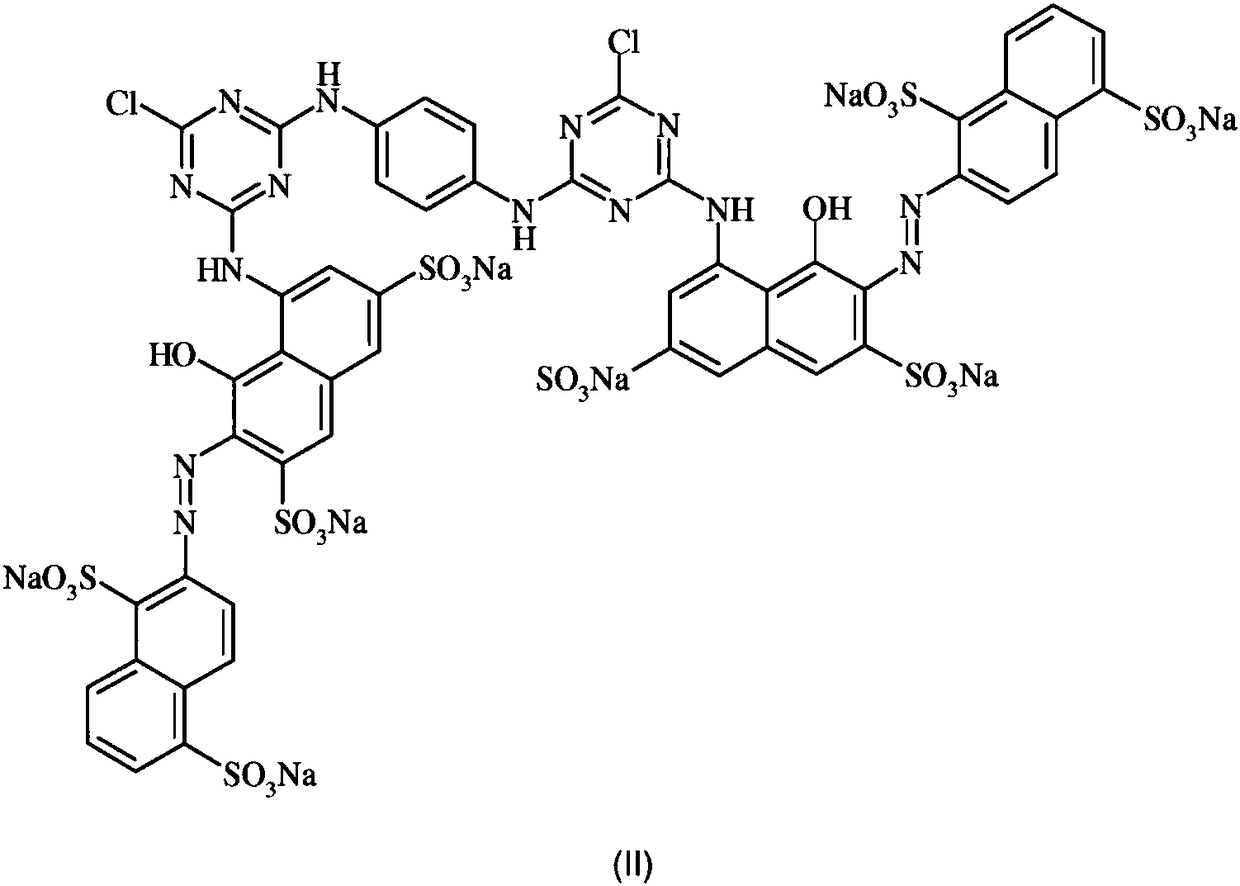
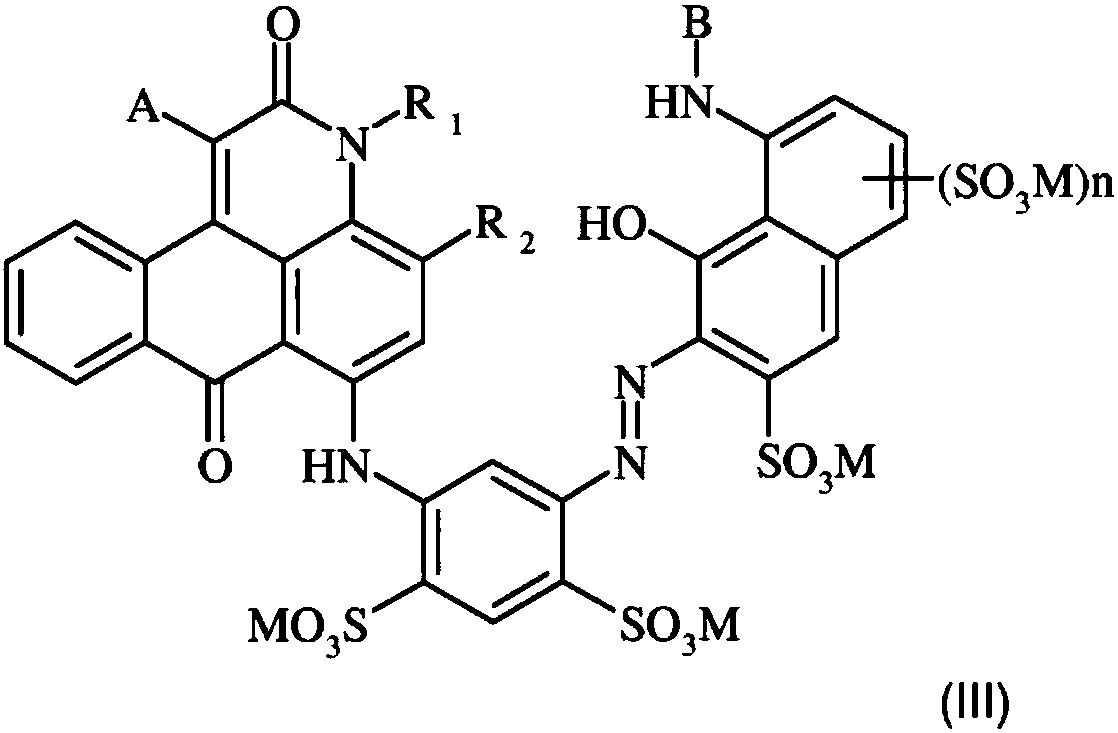
Anthrapyridone azo dyes, their preparation and use
An anthrapyridone azo, dye technology, applied in the direction of anthraquinone-azo dyes, azo dyes, anthracene dyes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051]Prepared dyes of formula (III), where M is designated, are listed in Table 1 .
[0052]
[0053]
[0054]
[0055]
[0056]
[0057]
[0058]
[0059]
[0060]
[0061]
[0062]
[0063]
[0064] The prepared dyes of structural formula (III) are listed in Table 1 together with their maximum absorption positions in aqueous solution (buffer pH 7.0):
[0065] Table 1
[0066]
[0067]
[0068] The compound of formula (III) may exist in the form of the free acid or its inorganic salt.
[0069] Preferably, these compounds are present in the form of their alkali metal or ammonium salts, where the ammonium cation may be substituted.
[0070] Examples of such substituted ammonium cations are 2-hydroxyethylammonium, bis-(2-hydroxyethyl)ammonium, tris-(2-hydroxyethyl)ammonium, bis-(2-hydroxyethyl)-methylammonium hydroxy-ammonium, tris-[2-(methoxyethoxy)-ethyl-ammonium, 8-hydroxy-3,6-dioxoctyl ammonium and tetraalkylammonium such as tetra...
Embodiment 1
[0095] Example 1: The anthrapyridone dyes (10A) of Table 1, where R = H and M is Na, were prepared in the following manner:
[0096] The preparation of the brominated anthrapyridone compound of structural formula (X):
[0097] 75.5g (0.25Mol) 1-amino-4-bromoanthraquinone (available from Sigma-Aldrich GmbH, Buchs, Switzerland), 59.5g (0.3Mol) ethyl benzoyl acetate 97% (available from Sigma-Aldrich GmbH, Buchs, Switzerland), 3.9 g (0.04 Mol) of potassium acetate and 125 ml of 1,2-dichlorobenzene were stirred at a temperature of 140° C. for 22 hours in a nitrogen atmosphere. About 12 ml of ethanol was removed from the reaction mixture by distillation.
[0098] Subsequently, the resulting dispersion was cooled to room temperature, the product was sucked off, washed well with ethanol and dried.
[0099] 70.2 g of product of formula (X) are obtained in this way.
[0100]
[0101] Preparation of dyes of structural formula (XI):
[0102] 34.4 g (0.08 Mol) bromoanthrapyridone...
Embodiment 2
[0117] Example 2: Anthrapyridone azo dyes (10B) in Table 1 were prepared as in Example 1, wherein M is Na. However, in the preparation of (X), the 1-amino-4-bromoanthraquinone (CAS81-62-9) in Example 1 was replaced with 1-methylamino-4-bromoanthraquinone (CAS 128-93-8) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com