Method for preparing three crystal configuration type calcium carbonate granules
A calcium carbonate and particle technology, applied in the direction of calcium carbonate/strontium/barium, etc., can solve the problems of complex preparation methods and difficult coexistence, and achieve the effects of high repeatability, strong operability, and strong potential economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
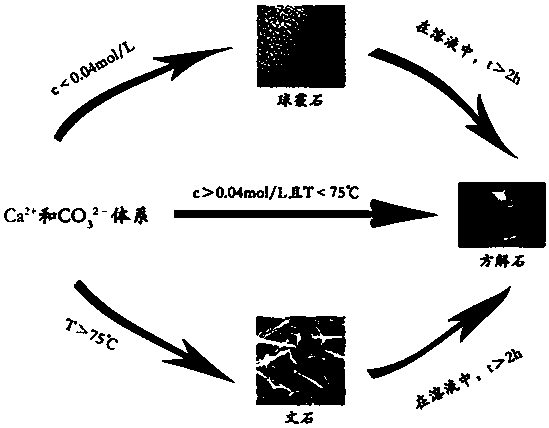
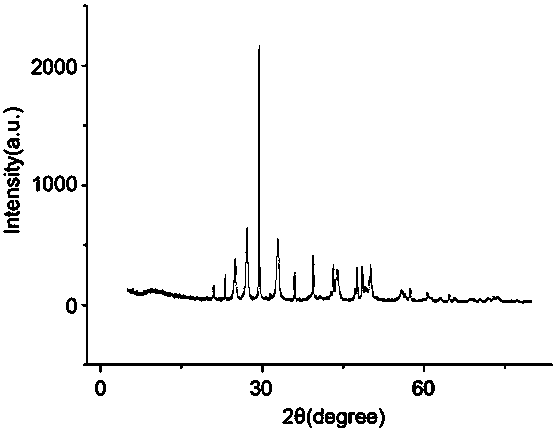
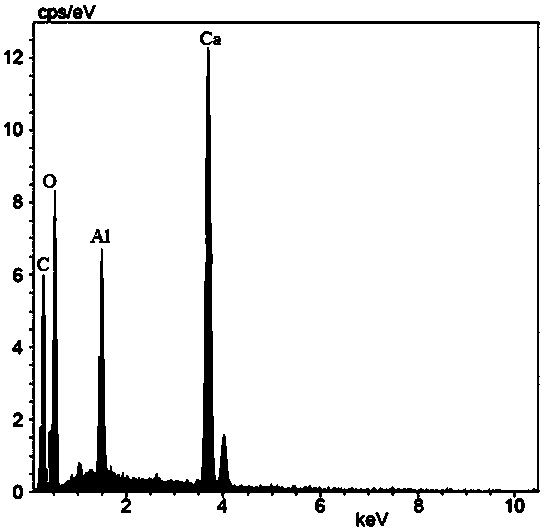
[0040] Carbonate ion (carbonate ion source is Na 2 CO 3 ) and calcium ions (the source of calcium ions is anhydrous CaCl 2 ) in a 1:1 equal concentration to prepare a salt solution system and place it in a 500mL beaker. After the saline solution system was ultrasonically oscillated for 10 min, the beaker was placed on a magnetic stirrer and magnetically stirred at a speed of 1000 rad / min. At 25°C, when the concentration of carbonate ions and calcium ions is 0.04mol / L and the reaction time is 1h, the salt solution system will generate vaterite-type calcium carbonate; the concentration of carbonate ions and calcium ions is 0.1mol / L, and the reaction temperature When the temperature is 75°C and the reaction time is 1.2h, the salt solution system will generate aragonitic calcium carbonate; when the reaction temperature is 25°C, the concentration of carbonate ions and calcium ions is 0.1mol / L, and the reaction time is 1h, the Salt solution systems generate calcite-type calcium c...
Embodiment 2
[0042] Carbonate ion (carbonate ion source is Na 2 CO 3 ) and calcium ions (the source of calcium ions is anhydrous CaCl 2 ) in a 1:1 equal concentration to prepare a salt solution system and place it in a 500mL beaker. After the saline solution system was ultrasonically oscillated for 10 min, the beaker was placed on a magnetic stirrer and magnetically stirred at a speed of 1000 rad / min. At 25°C, when the concentration of carbonate ions and calcium ions is 0.005mol / L and the reaction time is 1h, the salt solution system will generate vaterite-type calcium carbonate; the concentration of carbonate ions and calcium ions is 0.1mol / L, and the reaction temperature When the temperature is 95°C and the reaction time is 1h, the salt solution system will generate aragonitic calcium carbonate; when the reaction temperature is 25°C, the concentration of carbonate ions and calcium ions is 0.6mol / L, and the reaction time is 1h, the salt The solution system will generate calcite type ca...
Embodiment 3
[0044] Carbonate ion (carbonate ion source is Na 2 CO 3 ) and calcium ions (the source of calcium ions is anhydrous CaCl 2) in a 1:1 equal concentration to prepare a salt solution system and place it in a 500mL beaker. After the saline solution system was ultrasonically oscillated for 10 min, the beaker was placed on a magnetic stirrer and magnetically stirred at a speed of 1000 rad / min. At room temperature, when the concentration of carbonate ions and calcium ions is 0.01mol / L and the reaction time is 1h, the salt solution system will generate vaterite-type calcium carbonate; the concentration of carbonate ions and calcium ions is 0.1mol / L, and the reaction temperature is At 80°C and the reaction time is 1h, the salt solution system will generate aragonitic calcium carbonate; when the reaction temperature is 25°C, the concentration of carbonate ions and calcium ions is 0.2mol / L, the reaction time is 3h, the The system produces calcite-type calcium carbonate. The solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com