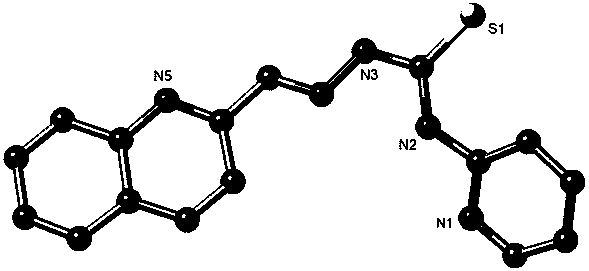
Quinoline thiocarbamide-pyridine organic compound and preparation method and application thereof
A kind of technology of quinoline thiosemicarbazide and organic compound, which is applied in the field of quinoline thiosemicarbazide-pyridine organic compound and preparation thereof, and can solve the problems of no synthesis method and application of quinoline thiosemicarbazide-pyridine organic compound and the like , to optimize biological properties, improve multi-targeting, and enhance inhibitory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation steps of quinoline thiosemicarbazone-pyridine organic compound are as follows:
[0024] (1) Weigh 2-aminopyridine (7.6 g, 0.08 mol) into a single-necked flask, add carbon disulfide (9.6 mL, 0.16 mol), triethylamine (24 mL, 0.16 mol), heat and stir the mixture in a water bath until clear , stirred at room temperature for 24 h. Treatment: Take the mixture in the flask and filter, and after obtaining the filter residue, use a small amount of methanol to completely dissolve it to reach a hot saturated solution. Then, slowly add a small amount of saturated methanol solution containing a large amount of product into a large amount of ether solution, and the yellow product quickly reappears in the ether solution. Intermediate product A crystallized out. Yield: 53%.
[0025] (2) Weigh the obtained yellow product A (12 g) into a round bottom flask, add methanol (25 mL) and methyl iodide (3 mL), and stir at room temperature for 1 h to obtain a reddish-brown reac...
Embodiment 2
[0031] The preparation steps of quinoline thiosemicarbazone-pyridine organic compound are as follows:
[0032] (1) Weigh 2-aminopyridine (7.6 g, 0.08 mol) into a single-necked flask, add carbon disulfide (4.8 mL, 0.08 mol), triethylamine (12 mL, 0.08 mol), heat and stir the mixture in a water bath until clear , stirred at room temperature for 12 h. Treatment: Take the mixture in the flask and filter, and after obtaining the filter residue, use a small amount of methanol to completely dissolve it to reach a hot saturated solution. Then, slowly add a small amount of saturated methanol solution containing a large amount of product into a large amount of ether solution, and the yellow product quickly reappears in the ether solution. Intermediate product A crystallized out. Yield: 44%.
[0033](2) Weigh the obtained yellow product A (10 g) into a round bottom flask, add methanol (20 mL), methyl iodide (2.5 mL), and stir at room temperature for 1 h to obtain a reddish-brown reacti...
Embodiment 3
[0037] The preparation steps of quinoline thiosemicarbazone-pyridine organic compound are as follows:
[0038] (1) Weigh 2-aminopyridine (7.6 g, 0.08 mol) into a single-necked flask, add carbon disulfide (7.2 mL, 0.12 mol), triethylamine (18 mL, 0.12 mol), heat and stir the mixture in a water bath until clear , stirred at room temperature for 18 h. Treatment: Take the mixture in the flask and filter, and add the filter residue to ether solution, stir and wash at room temperature for 10-20 min, filter, and dry the filter residue in air to obtain intermediate product A. Yield: 85%.
[0039] (2) Weigh the obtained yellow product A (10 g) into a round bottom flask, add methanol (35 mL) and methyl iodide (3 mL), and stir at room temperature for 1.5 h to obtain a reddish-brown reaction solution. Treatment: Slowly pour the reaction solution into about 600 mL of warm water, and white flocs precipitate out. After standing for 1.5 days, pale yellow needle-like crystals precipitate out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com