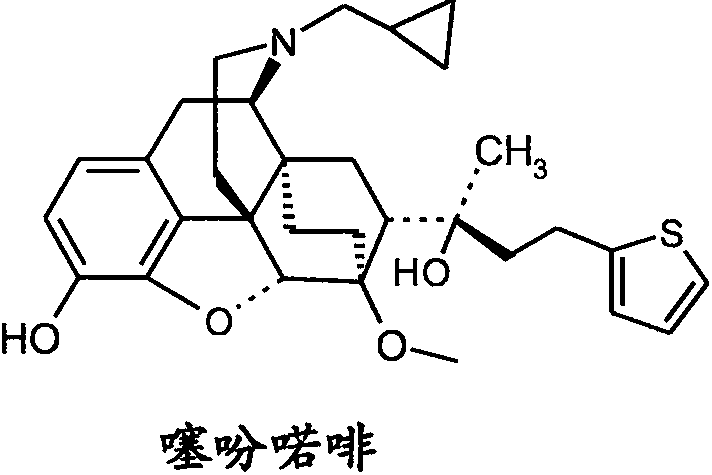
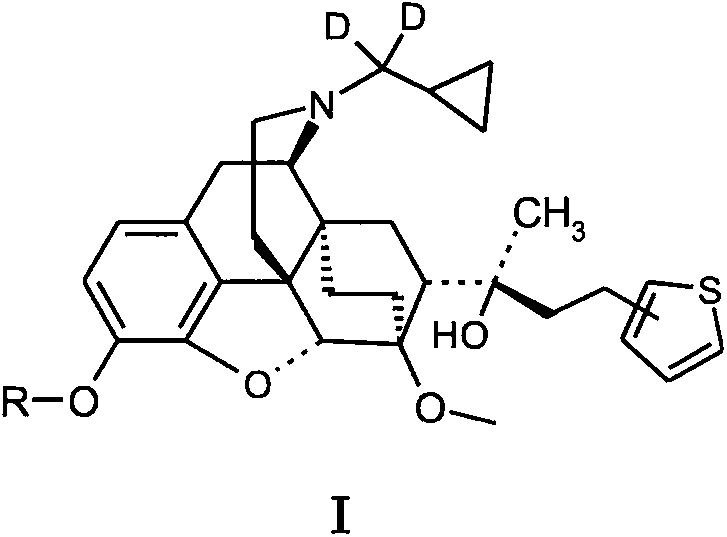
Novel and high efficiency anti-addiction drug
A drug and addiction technology, applied in the field of new high-efficiency anti-addiction drugs, can solve problems such as side effects, large individual differences of thienorphine, and limited clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] 1.7 Thienomorphine (C 1 ) preparation
[0029] 1.2g (2mmol) vii 1 Dissolve in 5ml of anhydrous tetrahydrofuran, add 2ml of 2M LiAlH dropwise under stirring 4 Tetrahydrofuran solution; after adding, stir overnight at room temperature; then add magnesium sulfate heptahydrate in batches until no gas is released. Filtrate, evaporate the filtrate to dryness under reduced pressure, separate by silica gel column chromatography, elute with petroleum ether: dichloromethane: methanol = 4: 1: 0.1, collect the required components, and recrystallize with methanol to obtain C 1 0.75g; Dissolve this solid with ethanol, add hydrogen chloride ether solution, to pH2, stir, precipitate solid, after standing overnight, filter and wash with anhydrous ether to obtain C 1 ·HCl 0.61g, melting point: >200°C. 1 H-NMR (400MHz, DMSO-d6): 9.62 (br, 1H); 9.39 (br, 1H); 7.26 (m, 1H); 7.01 (d, 1H); 6.91 (m, 1H); 1H); 6.55(d, 1H); 4.64(br, 1H); 4.54(s, 1H); 3.90(d, 1H); 3.41(s, 3H); 3.28(m, 2H); 3...
Embodiment 1
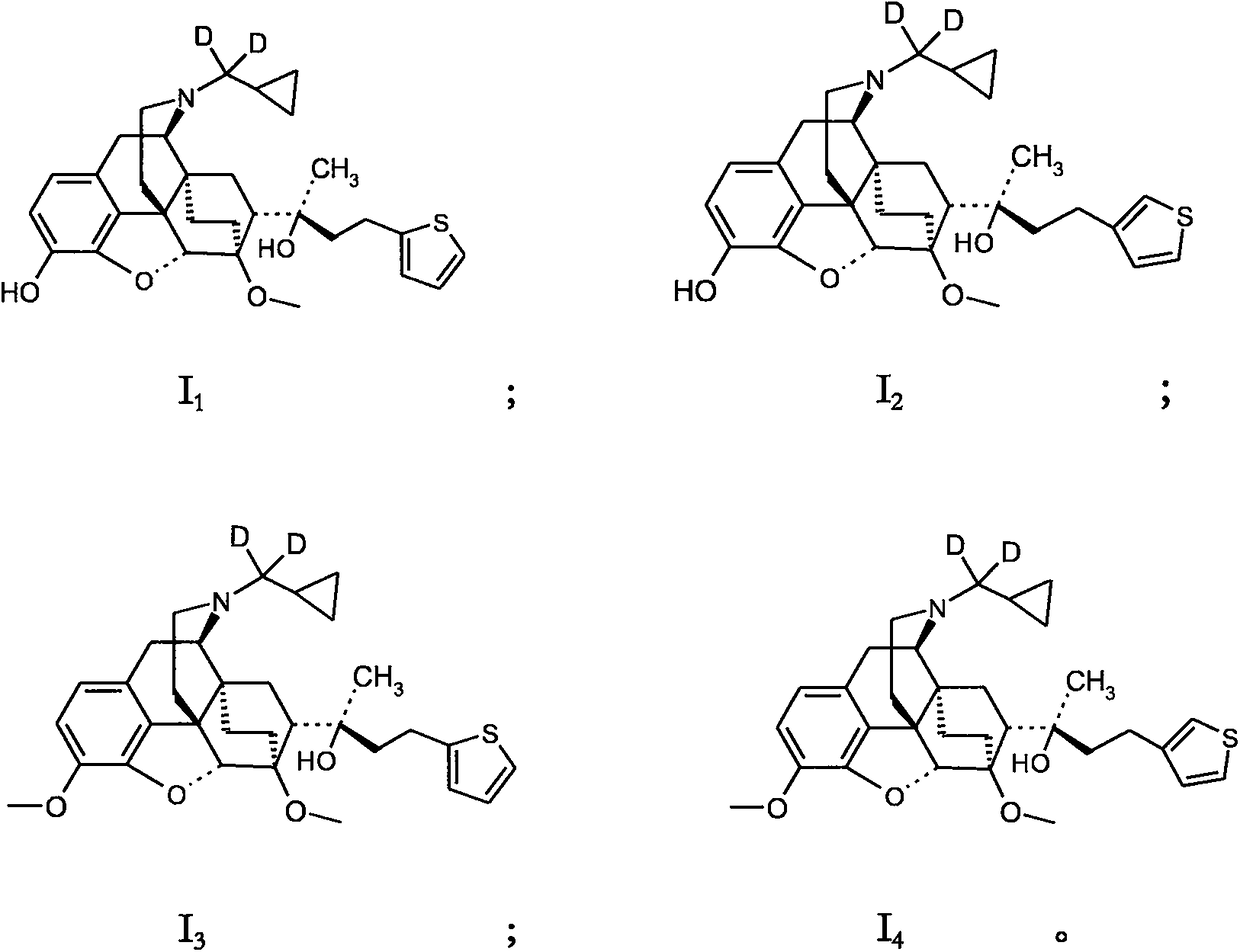
[0036] Example 1 (2S)-2-[(5R, 6R, 7R, 14S)-N-(cyclopropyl-dideuteriomethyl)-4,5-epoxy-6,14-ethylene- 3-Hydroxy-6-methoxymorphinan-7-yl]-4-(thiophen-2-yl)-butan-2-ol (I 1 ) preparation
[0037]
[0038] With reference to the method of reference example 1.7, vii 1 with LiAlD 4 (% D: > 98) reaction, makes I 1 ; will I 1 Dissolve in ethanol, form a salt with hydrogen chloride, and obtain I 1 · HCl, melting point: >200°C. 1 H-NMR (400MHz, DMSO-d6): 9.60 (br, 1H); 9.34 (br, 1H); 7.26 (m, 1H); 7.00-6.90 (m, 2H); 6.73 (d, 1H); d, 1H); 4.64(br, 1H); 4.54(s, 1H); 3.90(d, 1H); 3.30-2.30(m, 7H); 2.28(m, 1H); 1.96(m, 2H); 1.82 (m, 2H); 1.70(m, 1H); 1.51(m, 1H); 1.47(m, 1H); 1.35(m, 1H); 1.30(s, 3H); 1.13(m, 1H); 0.67( m, 2H); 0.58(m, 2H); 0.39(m, 1H).
Embodiment 2
[0039] Example 2 (2S)-2-[(5R, 6R, 7R, 14S)-N-(cyclopropyl-dideuteromethyl) 4,5-epoxy-6,14-ethylene-3 Preparation of -Hydroxy-6-methoxymorphinan-7-yl]-4-(thiophen-3-yl)-butan-2-ol ()
[0040]
[0041] With reference to the method of reference example 1.7, vii 2 with LiAlD 4 (% D: > 98) reaction, makes I 2 ; will I 2 Dissolve in ethanol, form a salt with hydrogen chloride, and obtain I 2 HCl, H NMR spectrum: 1 H-NMR (400MHz, DMSO-d6): 9.66 (br, 1H); 9.37 (br, 1H); 7.43-7.02 (m, 3H); 6.73 (d, 1H); 6.55 (d, 1H); 4.64 ( br, 1H); 4.54(s, 1H); 3.90(d, 1H); 3.28-2.79(m, 7H); 2.26(m, 1H); 1.95-1.70(m, 5H); 2H); 1.37(m, 1H); 1.32(s, 3H); 1.12(m, 1H); 0.36(m, 1H); 0.59(m, 2H); 0.68(m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com