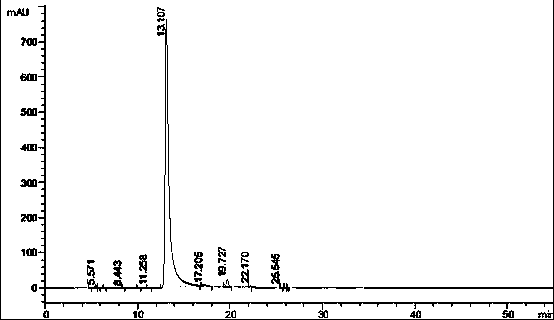
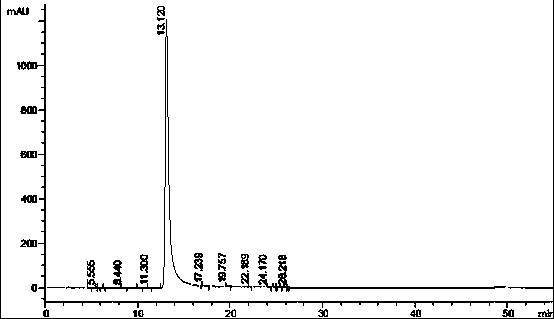
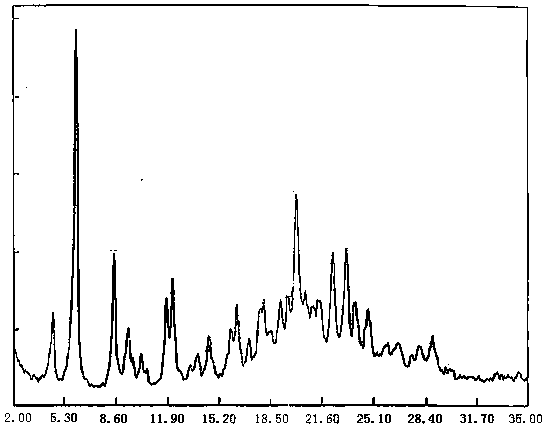
Bortezomib purification method
A technology of bortezomib and purification method, which is applied in the fields of chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problems of bortezomib with many impurities and reduce production costs, Good dissolution speed, saving manpower and material resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add a certain amount of crude bortezomib to an appropriate amount of ethyl acetate and heat to 60-65°C to dissolve, and prepare four solutions with concentrations of 300mg / mL, 90mg / mL, 60mg / mL and 30mg / mL respectively, and cool to 25°C Stand still for crystallization for 22 hours, filter to obtain the finished product of bortezomib, take a small amount for liquid phase detection of purity, and weigh the remaining sample after vacuum drying at 30-40°C to calculate the yield.
[0027] serial number
Embodiment 2
[0029] Add a certain amount of crude bortezomib to an appropriate amount of ethyl acetate and heat to 60-65°C to dissolve, and prepare a crystallization solution with a concentration of 60mg / mL, and select 40°C, 30°C, 20°C, 10°C and 0°C respectively Four different crystallization temperatures, static crystallization for 22 hours, filtered to obtain the finished product of bortezomib, a small amount was used for liquid phase detection of purity, and the remaining samples were vacuum-dried at 30-40°C and weighed to calculate the yield.
[0030] serial number
Embodiment 3
[0032] A certain amount of crude bortezomib was added to an appropriate amount of ethyl acetate and heated to 60-65°C to dissolve to prepare a crystallization solution with a concentration of 60mg / mL, and cooled to 20-30°C to stand for crystallization, and to investigate different crystallization times Effect of Team Bortezomib Yield.
[0033] serial number
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com