Preparation method of blood contact material for improving biocompatibility and blood contact material
A technology of biocompatibility and contact materials, which is applied in the field of biomedical functional materials, can solve the problems of unstable anticoagulant in vivo implantation and high incidence of adverse reactions, so as to improve biocompatibility, reduce incidence, good anticoagulant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
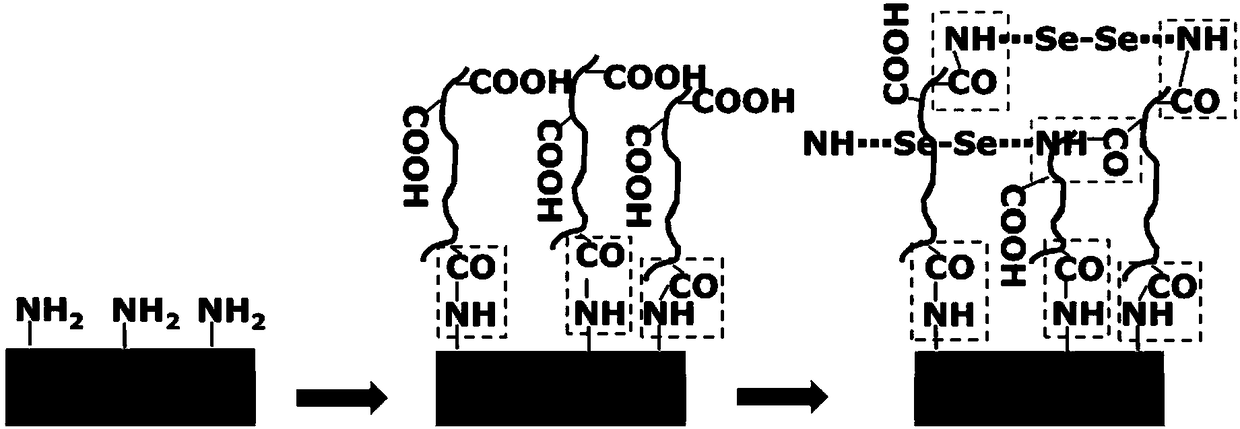
[0036] An embodiment of the present invention provides a method for preparing a blood contact material for improving biocompatibility, which includes the following steps:
[0037] S1 preparation activator: 2-(N-morpholine)ethanesulfonic acid (MES), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), N- Mix hydroxysuccinimide (NHS) and water to prepare the activator. Generally, a certain amount of MES solution is first prepared at a concentration of 40-60mmol / L, then a certain amount of EDC and NHS are added, shaken and mixed to prepare an activator, and the concentration of MES in the obtained activator is 40-60mmol / L. The concentration of EDC is 5-7mmol / L, and the concentration of NHS is 8-12mmol / L.
[0038] S2 modified hyaluronic acid: immerse the base material of amino-rich coating in the first mixed solution prepared by hyaluronic acid solution (HA solution) and activator for the first amide reaction, the first amide reaction is generally at 35 The amide r...
Embodiment 1
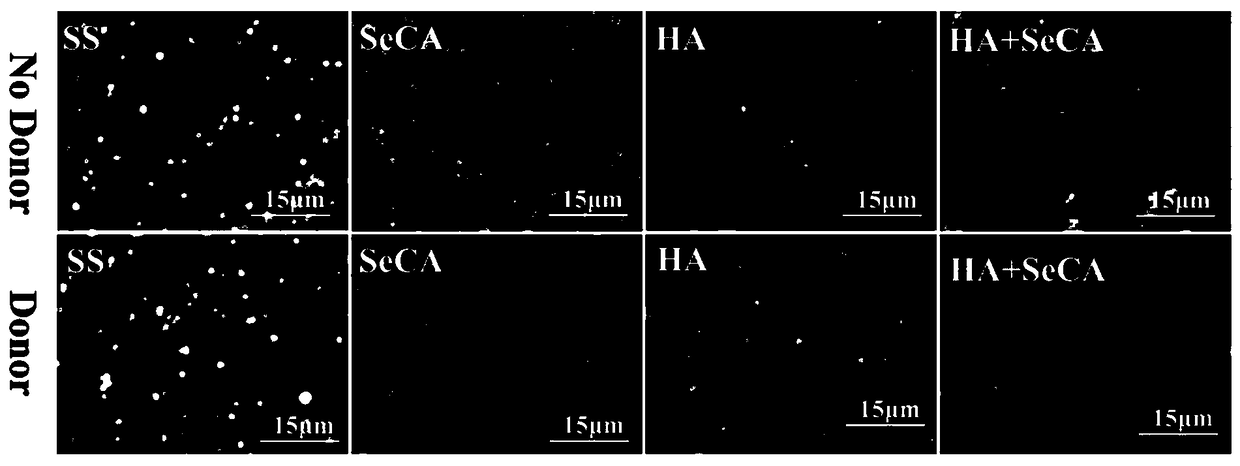
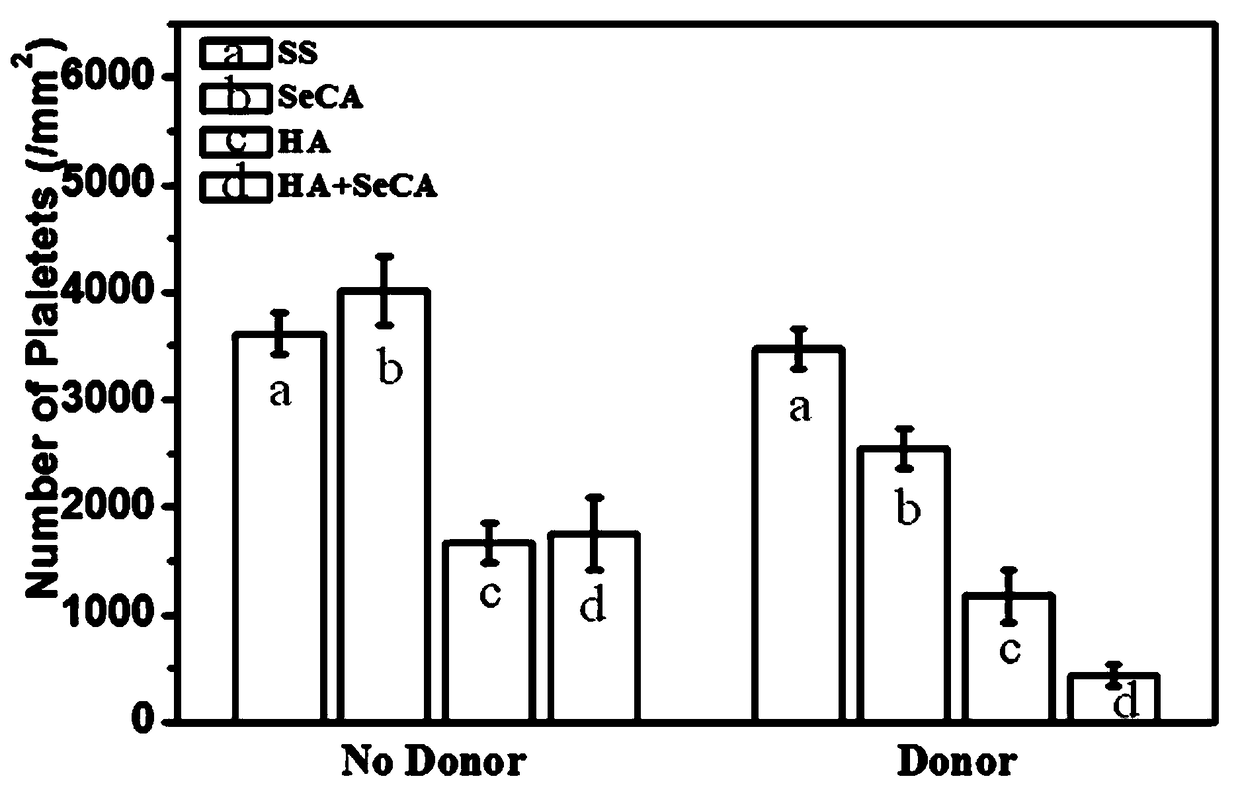
[0049] The embodiment of the present invention provides a blood contact material, which is modified with HA+SeCA, and it is prepared according to the following preparation method:
[0050] First prepare a certain amount of MES solution at a concentration of 50mmol / L, then add a certain amount of EDC and NHS, shake and mix to prepare an activator, the concentration of MES in the obtained activator is 50mmol / L, and the concentration of EDC is 6mmol / L The concentration of L and NHS is 10mmol / L.
[0051] According to the molar ratio of -COOH in the HA solution to EDC and NHS in the activator is 1:6:10, the material is taken out, the activator is added to the HA solution, and the first mixed solution is obtained after activation for 30 minutes. The substrate material was immersed in the first mixed solution for the first amide reaction at 37° C. for 24 hours, and then taken out and washed with deionized water for 3 times to obtain the HA modified material.
[0052] Prepare a SeCA ...
Embodiment 2
[0054] The embodiment of the present invention provides a blood contact material, which is modified with HA+SeCA, and it is prepared according to the following preparation method:
[0055] First prepare a certain amount of MES solution at a concentration of 40mmol / L, then add a certain amount of EDC and NHS, shake and mix to prepare an activator, the concentration of MES in the obtained activator is 40mmol / L, and the concentration of EDC is 7mmol / L The concentration of L and NHS is 12mmol / L.
[0056] According to the molar ratio of -COOH in the HA solution to EDC and NHS in the activator is 2:7:12, the material is taken out, the activator is added to the HA solution, and the first mixed solution is obtained after activation for 10 minutes. The base material was immersed in the first mixed solution for the first amide reaction at 35° C. for 40 hours, and then taken out and washed with deionized water for 3 times to obtain the HA modified material.
[0057] Prepare a SeCA solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com