Process for synthesizing hindered amine light stabilizer 944
A hindered amine light stabilizer and synthetic process technology, applied in the direction of organic chemistry, can solve the problems of rising cost, increasing post-processing difficulty, and reducing product quality, so as to reduce the possibility, reduce post-processing difficulty, and reduce by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of concrete steps of the synthesis technique of hindered amine light stabilizer 944 are as follows:
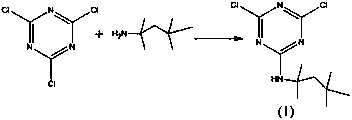
[0034] (1) Pour 92.3g of cyanuric chloride into the flask, add 185g of xylene, and drop 64.5g of tert-octylamine solution at 10°C for 6 hours. After 5 hours of heat preservation, slowly add 100g of 20% sodium hydroxide solution, the temperature is controlled at about 10°C, the dropwise addition is completed in 2 hours, and then kept for 6 hours. After the reaction was finished, 322g of Intermediate 1 solution (containing 137g of Intermediate 1) was obtained by cooling down and washing with water.
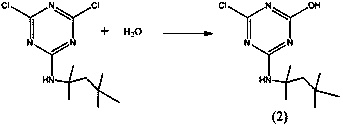
[0035] (2) Take 129g of Intermediate 1 solution (containing 54.8g of Intermediate 1) and pour it into a flask, add 5.5g of water, react at 40°C for 6h, cool down, separate water, filter, and precipitate to obtain 50g of Intermediate 2.
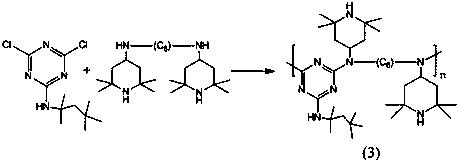
[0036] (3) Take 193g of intermediate 1 solution (containing 82.2g of intermediate 1) and pour it into the autoclave, and add 82.2g o...
Embodiment 2
[0039] A kind of concrete steps of the synthesis technique of hindered amine light stabilizer 944 are as follows:
[0040] (1) Pour 92.3g of cyanuric chloride into the flask, add 370g of toluene, drop 68g of tert-octylamine solution at 30°C for 11 hours, and slowly add 120g of 32 % sodium hydroxide solution, the temperature is controlled at about 30°C, the dropwise addition is completed in 6 hours, and then kept for 4 hours. After the reaction, the intermediate 1 solution was obtained by lowering the temperature and washing with water.
[0041] (2) Pour 200g of Intermediate 1 solution (containing 55.2g of Intermediate 1) into a flask, add 50g of water, react at 60°C for 4 hours, cool down, separate water, filter, and remove solvent to obtain 50.3g of Intermediate 2.
[0042] (3) Take 300g of intermediate 1 solution (containing 82.8g of intermediate 1) and pour it into the autoclave, add 200g of 15% sodium hydroxide solution and 158g of hexamethylenediamine piperidine in seque...
Embodiment 3
[0045] A kind of concrete steps of the synthesis technique of hindered amine light stabilizer 944 are as follows:
[0046] (1) Pour 92.3g of cyanuric chloride into the flask, add 554g of toluene, and drop 71g of tert-octylamine solution at 50°C for 15 hours. After 1 hour of heat preservation, slowly add 150g of concentration It is a 40% sodium hydroxide solution, the temperature is controlled at about 50°C, the dropwise addition is completed in 8 hours, and then kept for 2 hours. After the reaction, the intermediate 1 solution was obtained by lowering the temperature and washing with water.
[0047] (2) Take 276.5g of Intermediate 1 solution (containing 54.2g of Intermediate 1) and pour it into a flask, add 271g of water, react at 80°C for 2 hours, cool down, separate water, filter, and precipitate to obtain 50g of Intermediate 2.
[0048] (3) Take 417.7g of intermediate 1 solution (including 83g of intermediate 1) and pour it into the autoclave, add 415g of 5% sodium hydroxi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com