Method and detection kit for judging whether a solid tumor is suitable for immunotherapy
A technology for immunotherapy and detection reagents, applied in biochemical equipment and methods, microbial determination/inspection, measurement devices, etc., can solve problems such as immune system identification and tumor-killing ability evaluation, achieve objective response rate improvement, and broad application Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Detection of mutation load
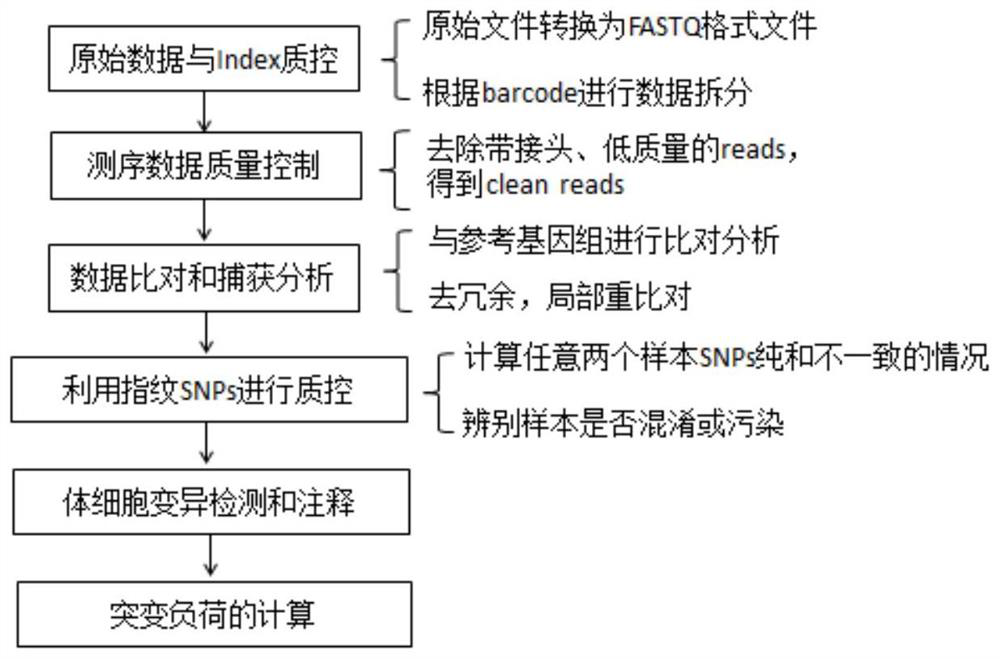
[0072] From April 2016 to November 2017, FFPE (formaldehyde-fixed paraffin-embedded) samples and peripheral blood of tumor tissues from 80 patients with solid tumors were collected. The peripheral blood cfDNA (circulating free DNA) and peripheral blood genomic DNA of these 80 patients with solid tumors were extracted, and the sequencing library was constructed, hybridized and captured, and sequenced on a computer, and bioinformatics analysis was performed on the obtained sequencing data. The process can be found in figure 1 . The tumor mutation burden of peripheral blood CtDNA was calculated according to the following formula 4.
[0073] Mutation load = total number of variations in the sample assay region / size of the target region (Formula 4)
Embodiment 2
[0074] Example 2 Detection of HLA heterozygous deletion
[0075] The tumor tissue and paired peripheral blood genomic DNA of 80 solid tumor patients described in Example 1 were extracted, and seven polymorphic STR sites located in the genomic region where the HLA gene was located were selected, and the seven STRs were detected by fluorescent PCR-capillary electrophoresis The homozygous status of the loci (7 loci are D6S2852, D6S2872, D6S248, D6S1022, D6S265, D6S273, D6S1666), and the heterozygous deletion ratio of each locus is calculated according to the following formula 5. In formula 5, allele 1 is the allele with lower peak height. If the heterozygous deletion ratio is higher than 0.4, it is considered that the heterozygous deletion occurs at this site. Compared with the peripheral blood genomic DNA, if there is a heterozygous deletion at one site, then The HLA status of this sample is heterozygous deletion.
[0076] Heterozygous deletion ratio=1-Ht value=1-[peak height o...
Embodiment 3
[0079] Example 3 Calculate the mRNA expression scores of 18 genes related to T cell inflammation
[0080] RNA was extracted from the tumor tissues of 80 patients with solid tumors in Example 1, and 18 genes related to T cell inflammation (see Table 1) were selected, reverse-transcribed into cDNA, and a fixed amount of external standard was added to the cDNA sample.
[0081] The Ct value of 18 genes related to T cell inflammation, 1 internal reference gene β-actin, and 1 external standard was detected by Q-RT-PCR method (based on taqman probe), and the expression score of each gene was calculated. Then calculate the average value, which is the mRNA expression score of the 18 T cell inflammation-related genes. The calculation method is described below taking the CXCR6 gene as an example: Absolute expression level of CXCR6=absolute copy number of external standard product*2 -ΔΔCt , while ΔΔCt=[Ct(CXCR6)-Ct(internal reference gene)]-[Ct(external standard)-Ct(reference gene)], the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com