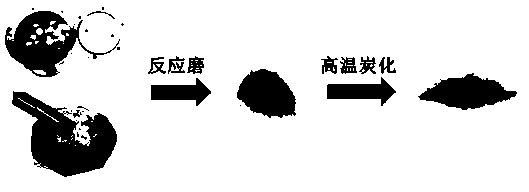
Method for preparing covalent organic polymer by reaction mill and application of covalent organic polymer
A covalent organic and polymer technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, carbon preparation/purification, etc., can solve the problem of low synthesis efficiency, pollution, large toxic and harmful solvents and other problems, to achieve the effect of short reaction time, low energy consumption and rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
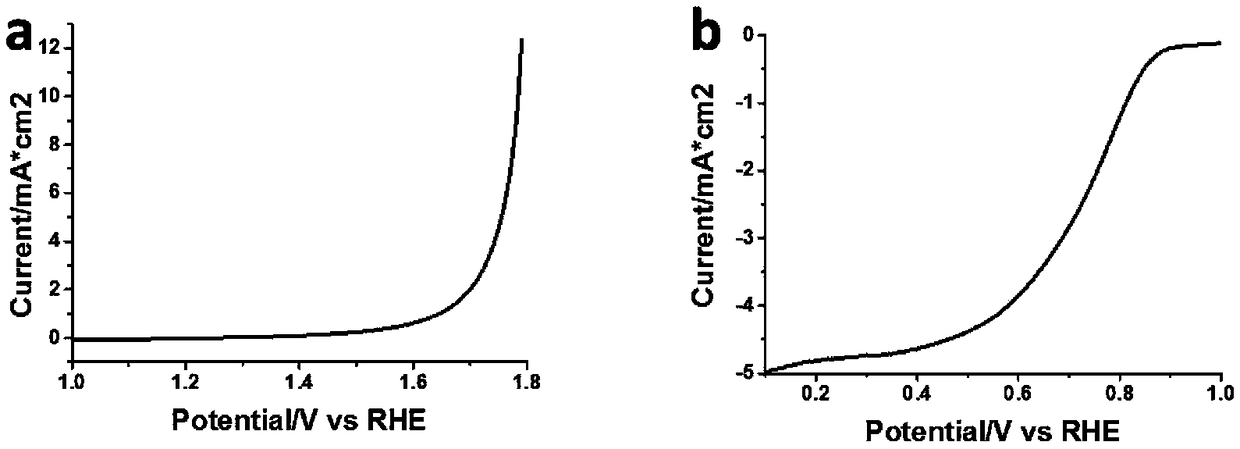
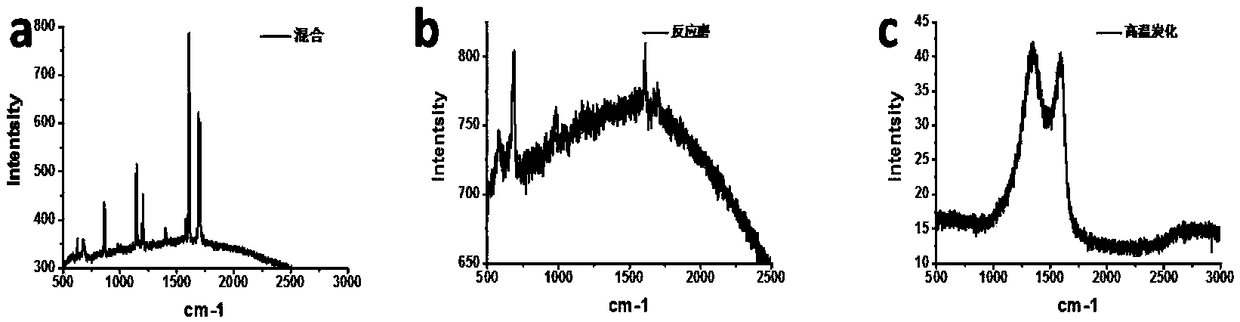
[0025] Such as Figure 4 As shown, weigh 1.5g of terephthalaldehyde and 1.5g of melamine respectively, then add 2ml of ethanol, grind and mix to a slurry state, put them in the reaction mill jar, the total volume of the reaction monomer is 2 / 5 of the volume of the reaction mill jar , reaction mill for 1 hour, the grinding frequency was kept at 600 rpm, the reaction mill medium was zirconia balls with a diameter of 2 mm, and the covalent organic polymer obtained after the reaction mill was carbonized at high temperature in a tube furnace, and the high temperature carbonization program was 10°C / The heating rate of min is to raise the temperature to 300°C and stay for 1 hour, then raise the temperature to 900°C at a heating rate of 10°C / min, and stay at 900°C for 2 hours. After carbonization, the final product, that is, a derivative of a covalent organic polymer, is obtained. The final product was electrochemically tested to obtain the OER and ORR performance of the catalyst, as...
Embodiment 2
[0027] Such as Figure 4 As shown, weigh 2g of terephthalic acid and 1.5g of melamine respectively, then add 1ml of N, N-dimethylformamide, grind and mix to a slurry state, and place them in a reaction mill jar. The total volume of the reaction monomer is the reaction mill 1 / 2 of the volume of the tank, the reaction mill is 30h, the grinding frequency is kept at 100 rpm, the reaction mill medium is zirconia balls with a diameter of 10mm, and the covalent organic polymer obtained after the reaction mill is carbonized at high temperature in a tube furnace. The carbonization program is a heating rate of 1°C / min, heating up to 500°C, staying at 500°C for 1 hour, rising to 700°C at a heating rate of 1°C / min, staying at 700°C for 5 hours, and obtaining the final product after carbonization, that is, covalent Derivatives of organic polymers. The final product was electrochemically tested to obtain the OER and ORR performance of the catalyst, and the OER was measured at 10mA / cm 2 Th...
Embodiment 3
[0029] Such as Figure 4 As shown, weigh 2 g of isophthalonitrile and 1.5 g of ZnCl 2 , grind and mix evenly, place in the reaction mill jar, the total volume of the reaction monomer is 3 / 4 of the volume of the reaction mill jar, the reaction mill is 5h, the grinding frequency is kept at 300 rpm, and the reaction mill medium is a circle with a diameter of 5mm Beads, the covalent organic polymer obtained after the reaction mill was washed with water to remove zinc chloride, and then carbonized at a high temperature in a tubular furnace. The high-temperature carbonization program was a heating rate of 8°C / min. The heating rate of °C / min was increased to 1100 °C, and the temperature was kept at 1100 °C for 4 hours. After carbonization, the final product, that is, the derivative of the covalent organic polymer, was obtained. The final product is electrochemically tested to obtain the OER and ORR performance of the catalyst, and the OER of 10mA / cm 2 The corresponding voltage is 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com