Drug composition for treating encephaledema by taking intracellular osmotic pressure as target
A cerebral edema and drug technology, applied in the field of medicine, can solve the problems of limited effect and inaccurate curative effect, and achieve the effect of maintaining stability and treating cerebral edema.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
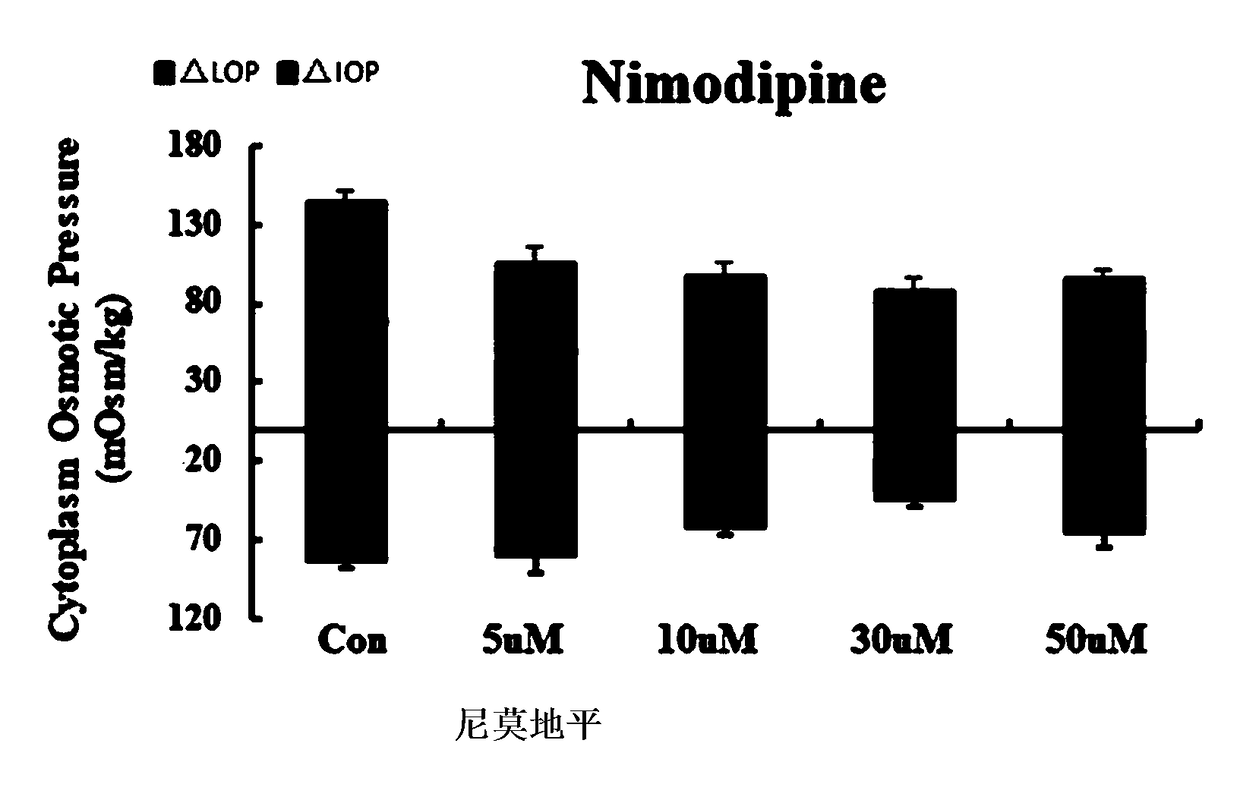
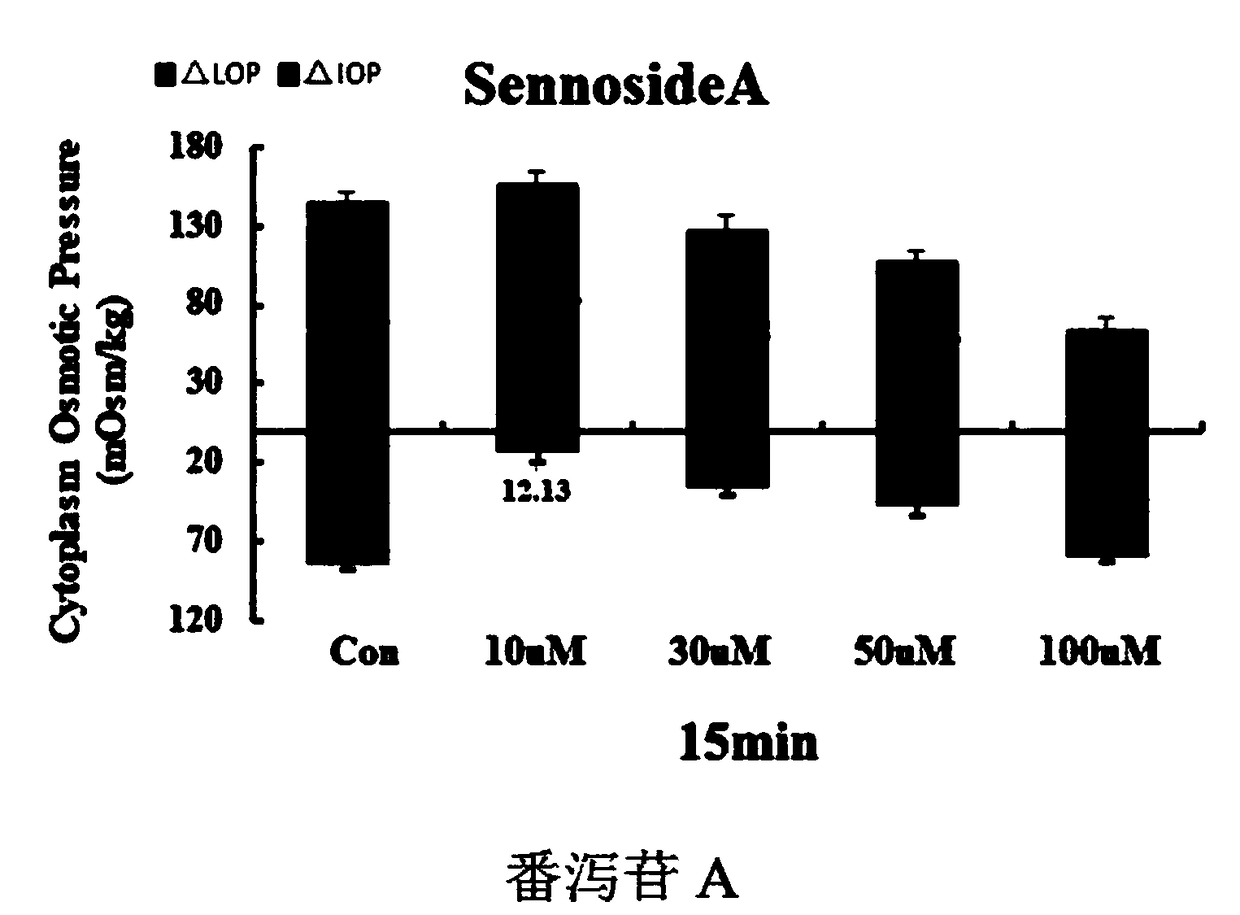
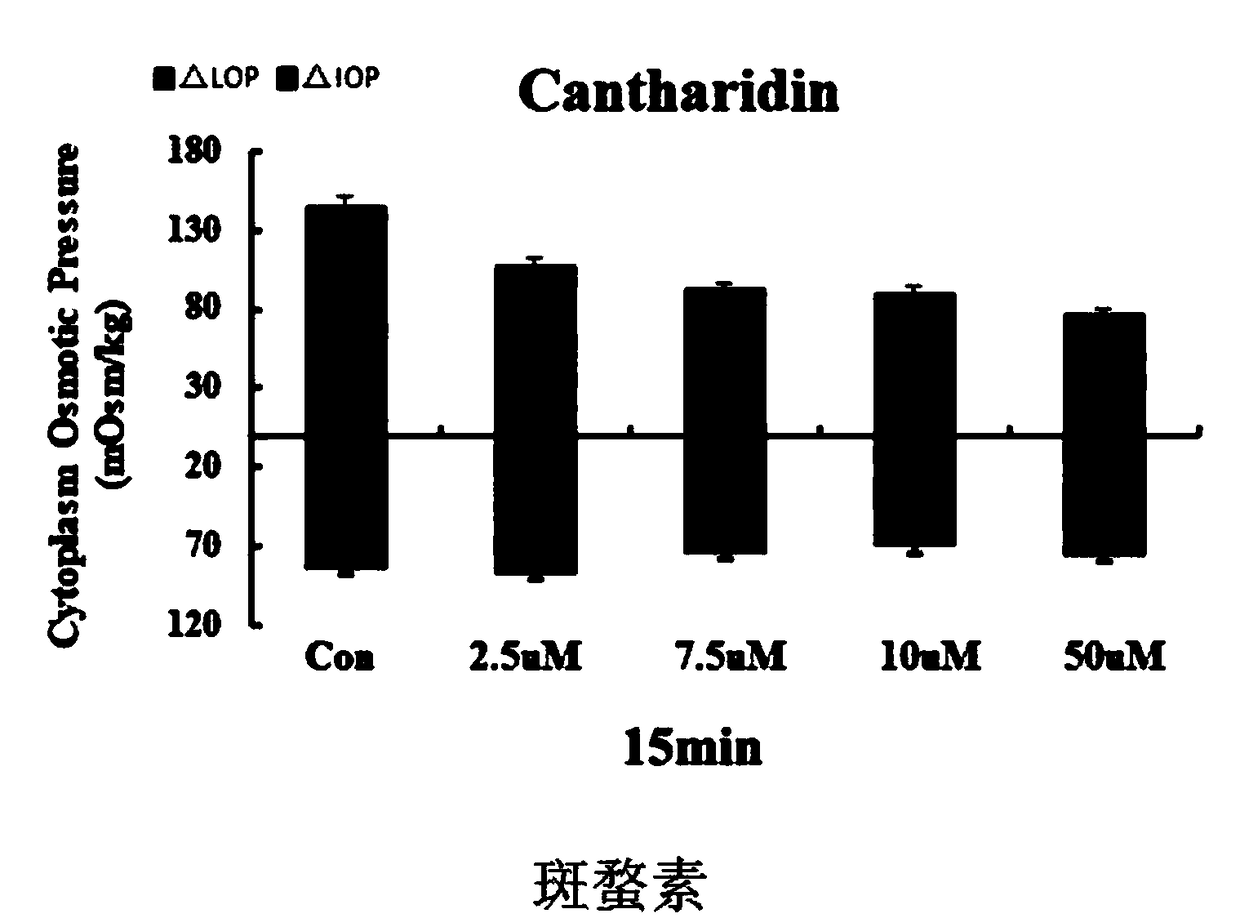
[0041]A compound pharmaceutical composition with the effect of treating cerebral edema, comprising the following raw materials in parts by weight (Mol): 100 parts of sennoside A, 2 parts of cantharidin, 30 parts of nimodipine, cinepazide maleate 7.5 copies and 50 copies of Edaravone.
[0042] A preparation method of a compound pharmaceutical composition with the effect of treating cerebral edema, comprising the following steps:
[0043] (1) Take each raw material according to the concentration ratio in the aseptic operation room, and set aside;
[0044] (2) Grinding sennoside A, cantharidin, nimodipine, and cinepazide maleate into powders and mixing uniformly at a temperature of 25-35°C to obtain mixture A;
[0045] (3) Grinding Edaravone into powder and mixing uniformly at a temperature of 30-35°C to obtain mixture B;
[0046] (4) Mix the above-mentioned mixture A and mixture B evenly, put them at a temperature of 5-15° C. and let them stand for 20-30 minutes to obtain the ...
Embodiment 2
[0051] A compound pharmaceutical composition with the effect of treating cerebral edema, comprising the following raw materials in parts by weight (Mol): 50 parts of sennoside A, 2 parts of norcantharidin, 20 parts of nimodipine, cinnamon maleate Chite 10 parts and Edaravone 10 parts.
[0052] A preparation method of a compound pharmaceutical composition with the effect of treating cerebral edema, comprising the following steps:
[0053] (1) Take each raw material according to the concentration ratio in the aseptic operation room, and set aside;
[0054] (2) Grinding sennoside A, norcantharidin, nimodipine, and cinepazide maleate into powders and mixing them uniformly at a temperature of 25-35°C to obtain mixture A;
[0055] (3) Grinding Edaravone into powder and mixing uniformly at a temperature of 30-35°C to obtain mixture B;
[0056] (4) Mix the above-mentioned mixture A and mixture B evenly, put them at a temperature of 5-15° C. and let them stand for 20-30 minutes to ob...
Embodiment 3
[0060] A compound pharmaceutical composition with the effect of treating cerebral edema, comprising the following raw materials in parts by weight (Mol): 75 parts of sennoside A, 2 parts of cantharidin, 25 parts of nifedipine, cinepazide maleate 5 parts and edaravone 15 parts.
[0061] A preparation method of a compound pharmaceutical composition with the effect of treating cerebral edema, comprising the following steps:
[0062] (1) Take each raw material according to the concentration ratio in the aseptic operation room, and set aside;
[0063] (2) Grinding sennoside A, cantharidin, nifedipine, and cinepazide maleate into powders and mixing them uniformly at a temperature of 25-35°C to obtain mixture A;
[0064] (3) Grinding Edaravone into powder and mixing uniformly at a temperature of 30-35°C to obtain mixture B;
[0065] (4) Mix the above-mentioned mixture A and mixture B evenly, put them at a temperature of 5-15° C. and let them stand for 20-30 minutes to obtain the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com