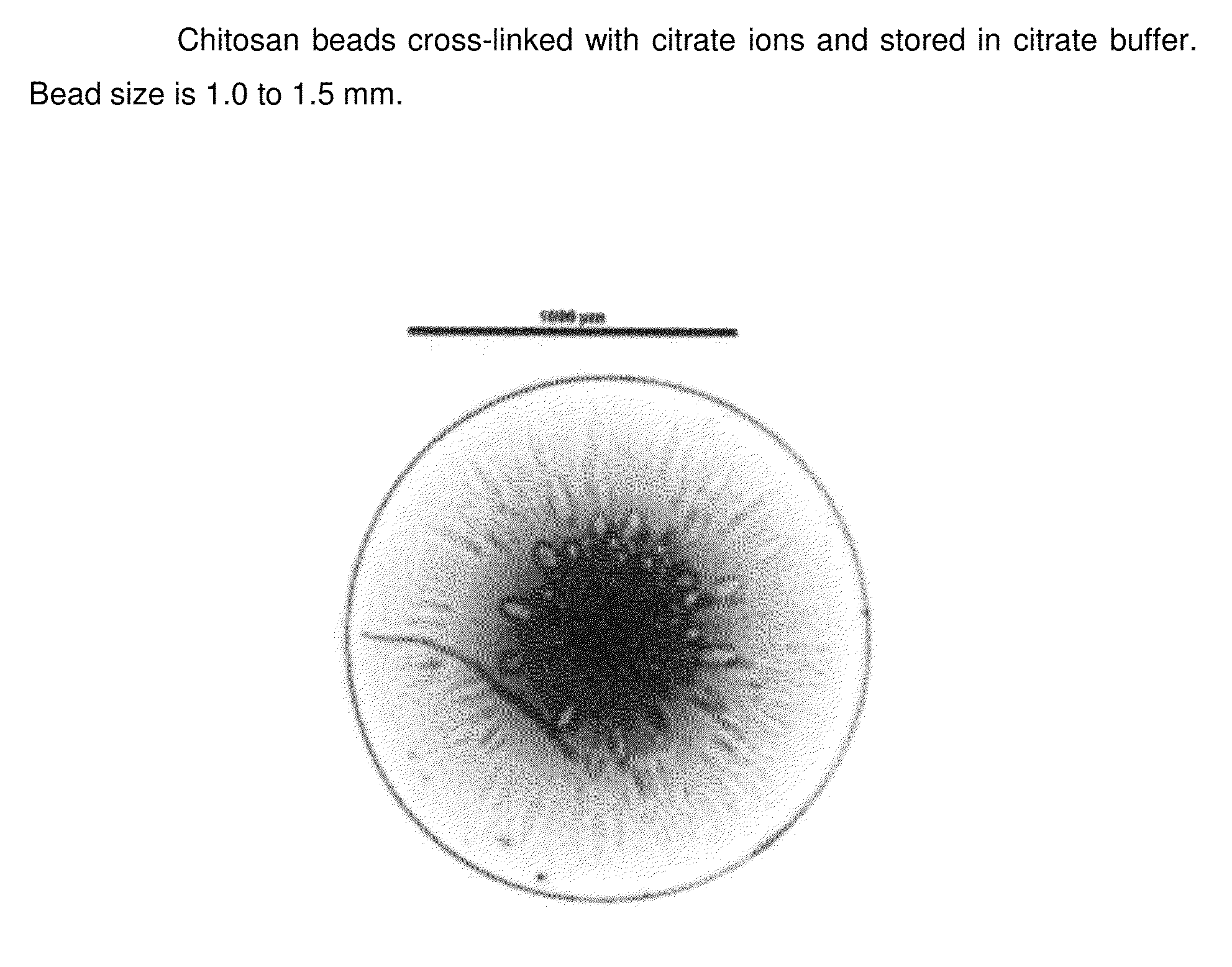
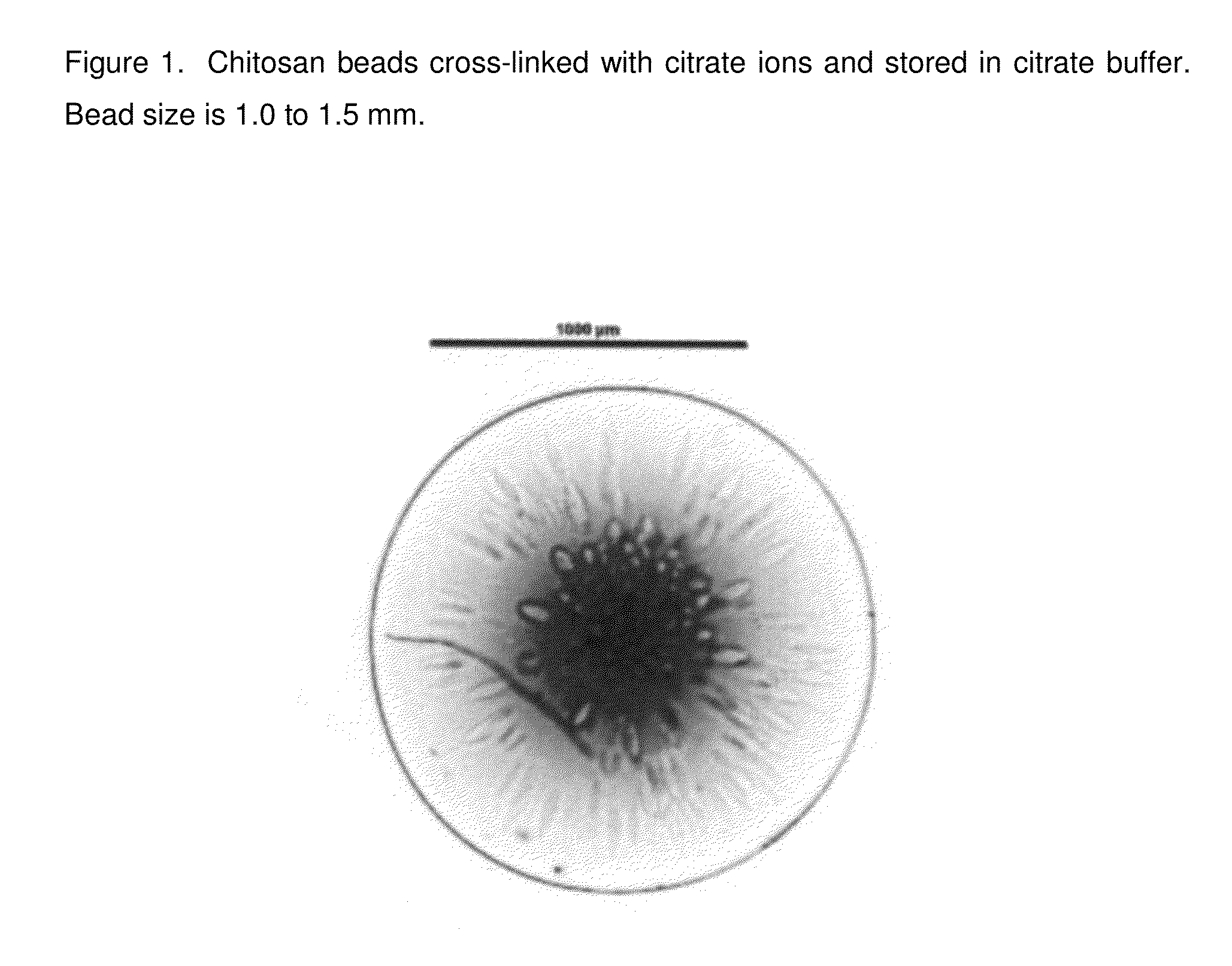
Chitosan beads and filler comprising such beads
a technology of chitosan beads and fillers, which is applied in the field of chitosan beads, can solve the problems of irregular microparticle formation, poor syringeability, and high cost of collagen and hyaluronic acid based fillers treatment, and achieves the effects of long-lasting effect, easy degradation, and rapid regrowth of volum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Manufacturing of Chitosan Beads and / or a Filler Comprising such Beads
[0110]Chitosan (Chitopharm L) is dispersed in deionized water. The appropriate amount of acid is added to the dispersion under constant stirring (0.05 mol per 10 g of chitosan). The dispersion is stirred until a clear solution is obtained. Having obtained a clear solution the pH of the obtained chitosan solution is stabilized from pH 1 to 5. With the help of a 30 Gauge syringe the chitosan solution is dropped into the following solutions having a pH from 5 to 11 and the obtained beads are let for 1 h in solution.
Crosslinking media1M Citrate1M citrate1M citrate1M citratepH of the media891011ObservationFormation ofFormation ofFormation ofFormation ofafter droppingcircular beadscircular beadscircular beadscircular beadsObservationElastic, flexibleElastic, flexibleLess flexible,Less flexible,after 1 h crosslinkingbeadsbeadsmore rigidmore rigidbeadsbeads
[0111]The obtained chitosan beads were investigated by employing a ...
example 2
Loading of Chitosan Beads with Active Pharmaceutical Ingredients
[0112]Chitosan is dispersed in deionized water. The appropriate amount of acid is added to the dispersion under a constant stirring. The dispersion is stirred until a clear solution is obtained. As a second solution, lidocaine hydrochloride USP (2% weight per weight) is dissolved in the chitosan solution. With the help of a 30 Gauge syringe the chitosan lidocaine solution is dropped into the following solutions having a pH from 1 to 5 and the obtained beads with lidocaine encapsulated are let for 1 h in solution.
example 3
Administration of the Filler Comprising Chitosan Beads
[0113]The filler prepared according to example 1 is injected to a 50-year old female patient into nasolabial folds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com