Techniques for predicting, detecting and reducing aspecific protein interference in assays involving immunoglobulin single variable domains
A technology of immunoglobulin and structural domain, applied in the field of immunoglobulin single variable domain, which can solve the problem of not providing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0231] Example 1: Generation of Antibodies for Polyclonal Analysis.
[0232] Polyclonal antibodies (IgG fraction) that can be used as "analytical antibodies" are produced as follows:
[0233] a. Identification of suitable plasma samples for isolation of polyclonal antibodies
[0234] Twenty plasma samples from healthy individuals who had never been treated with ISV were evaluated for the presence of antibodies against ISV useful as analytical antibodies of the present invention.
[0235] The ISV used initially in this example was SEQ ID NO:1. Subsequently, the assay described below was repeated with other ISV's in order to confirm that the interaction was not specific to this particular ISV, but rather a non-specific protein-protein interaction that may occur with many ISV's (see paragraph C) below). As an alternative to SEQ ID NO: 1, eg SEQ ID NO: 2 can also be used.
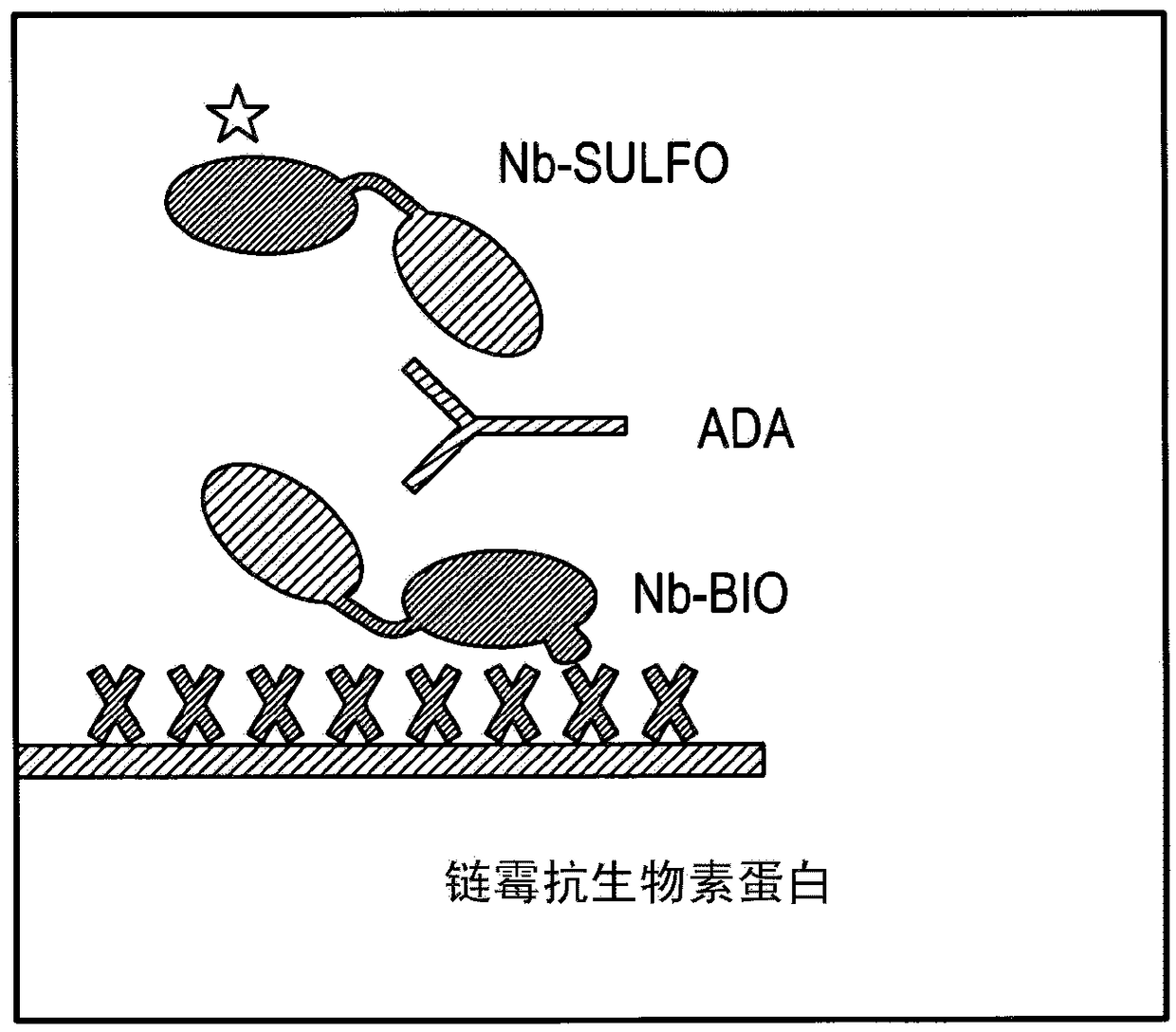
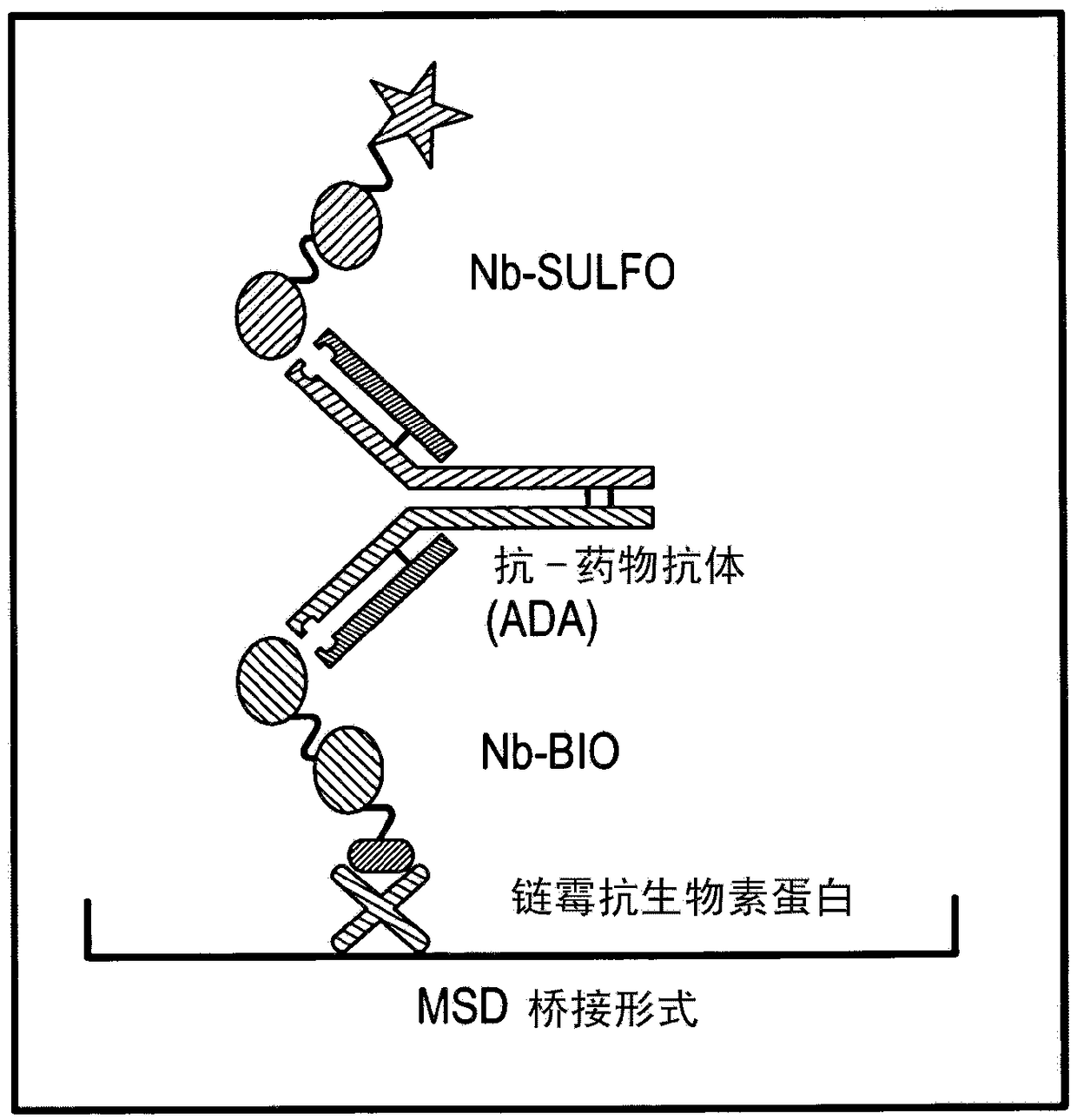
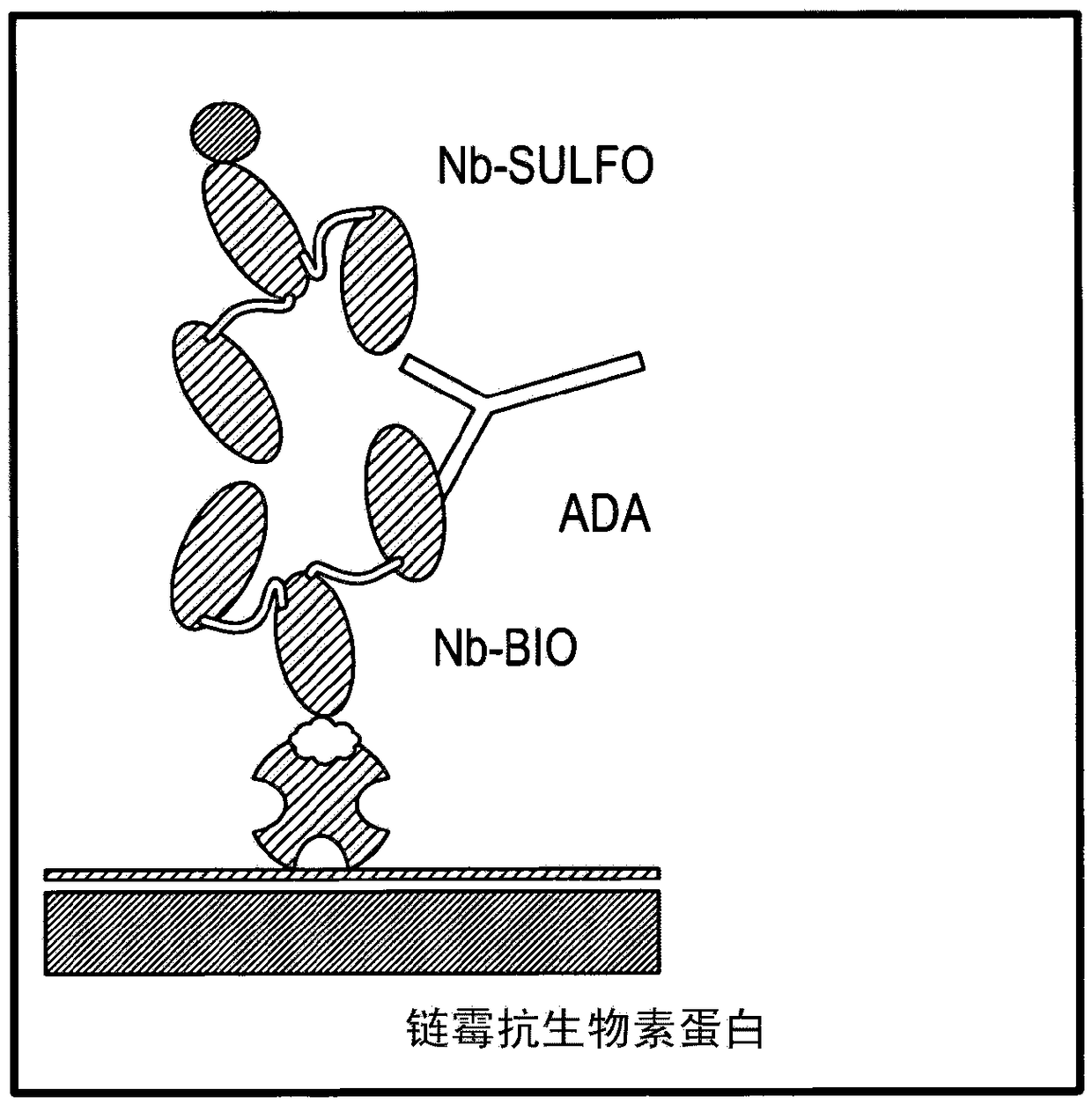
[0236] The assay used was an ECL (electrochemiluminescence) based bridging assay using biotinylated ISV...
Embodiment 2
[0260] Example 2: Analysis of affinity purification of antibodies
[0261] This example describes two methods that can be used to isolate analytical antibodies capable of recognizing and / or binding to the C-terminal end of ISV from biological fluids from human subjects. Antibodies were isolated from 4 different serum samples characterized in that these induced a positive signal in the ADA assay tested according to Example 1.
[0262] Starting from a serum sample, each of these methods provides a purified preparation of an interfering factor that can be used as an analytical antibody in the methods described herein. These methods can also be used, more generally, to purify interfering factors for other purposes (for example, interfering factors purified using the protocol below were also used experimentally in Example 8 to show that ISV or ISV-constructs were associated with monoclonal 21-4 Binding of the same ISV or ISV-construct with interfering factors, and thus predicts ...
Embodiment 2A
[0263] Example 2A: Purification Using Protein A and Affinity Chromatography
[0264] In the first step, IgG antibody fractions are enriched from serum samples using protein A affinity chromatography. Typical columns used for this enrichment include HiTrap MabselectSure and MabSelectXtra (GE Healthcare); PorosMabCapture A (Applied Biosystems). In all experiments, IgG antibodies were purified from serum samples in an automated and analogous manner. Chromatographic protocols were performed on an AKTA purification system (GE Healthcare) and recorded in real time using UNICORN protein purification software (GE Healthcare). Briefly, serum samples were diluted 1:1 with D-PBS (Dulbecco's Phosphate Buffered Saline) and filtered at 0.22 μm before loading onto the column at a fixed flow rate of 0.5 mL / min. The column was washed with D-PBS at a flow rate of 0.5 mL / min to remove non-specifically bound components within 5 column volumes. The IgG fraction was eluted by acidic elution us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com