Preparation methods for hydrophobic alcohol metal compound and isosorbide-modified polyester
A technology of metal compound and isosorbide is applied in the field of preparation of modified polyester to achieve stable performance, reduce loss of raw materials, and shorten reaction time of esterification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
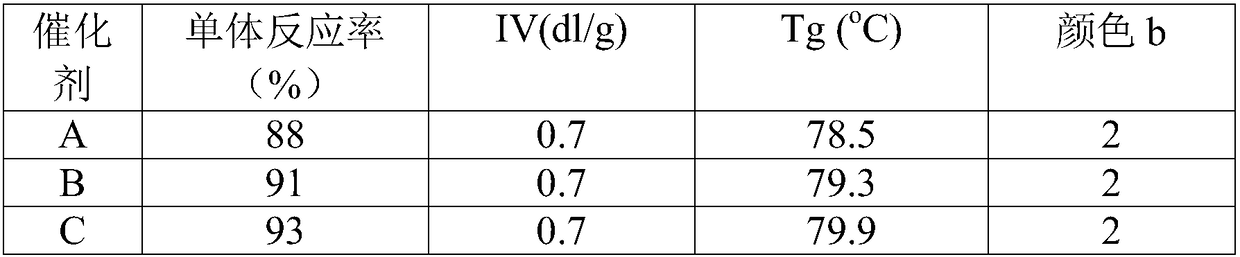
[0020] Catalyst A preparation
[0021] The preparation of described catalyst A can operate according to the following steps:
[0022] First, add 31.5g of BiCl in a 500mL three-neck flask under stirring 3 with 9.49gTiCl 4 (BiCl 3 with TiCl 4 The molar ratio is about 2:1) mix evenly, slowly add 19.53g of n-octanol under stirring, absorb the generated acid gas with lye, age at room temperature for 2 hours, after no gas is released, stir at 120°C for 3 hours, Catalyst A was prepared.
Embodiment 2
[0024] Catalyst B preparation
[0025] The preparation method of catalyst B is the same as that of catalyst A in Example 1, except that the BiCl used 3 with TiCl 4 The molar ratio is about 1:1.
Embodiment 3
[0027] Catalyst C preparation
[0028] The preparation method of catalyst C is the same as the preparation of catalyst A in Example 1, except that the used BiCl 3 with TiCl 4 The molar ratio is about 1:2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com