Herba cistanche, herba cistanche extracted product, and applications of herba cistanche total glycoside extract product in cardiac neurosis
A technology of Cistanche deserticola extract and neurosis, which can be used in nervous system diseases, medical preparations containing active ingredients, drug combinations, etc., and can solve the problems of lack of proper exercise in the circulatory system.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
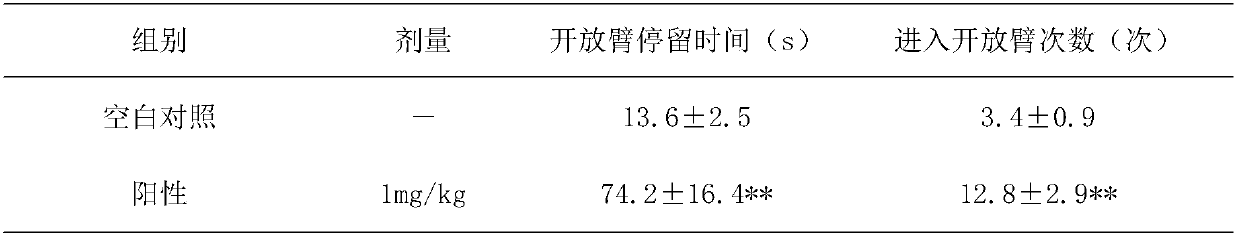
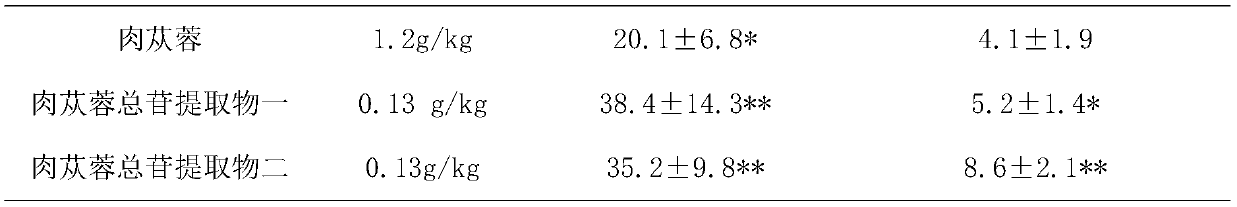
[0022] Example 1: Invention of test substances on anxiety-like behavior in mice
[0023] 1. Experimental materials
[0024] 1.1 Drugs and reagents
[0025] Tested drugs: commercially available Cistanche medicinal materials, Cistanche total glycosides extract I (Example 5), Cistanche total glycosides extract II (Example 6);
[0026] Diazepam, Tianjin Lisheng Pharmaceutical Co., Ltd.
[0027] 1.2 Animals
[0028] ICR mice, weighing 18-22 g, male, SPF grade, provided by Beijing Weitong Lihua, license number: SCXK (Beijing) 2016-0011.
[0029] 1.3 Instruments
[0030] Rat and mouse elevated plus maze video experiment system, Shanghai Ruanlong Technology Development Co., Ltd.
[0031] 2. Experimental method
[0032] 50 male ICR mice were randomly divided into 5 groups according to body weight, including 10 blank control groups (administered with the same volume of purified water), 10 positive control groups (diazepam 1mg / kg), 2 groups of test drugs: Cistanche deserticola Her...
Embodiment 2
[0044] Embodiment 2: Research on the anti-fatigue effect of the invented test substance on mice
[0045] 1. Experimental materials
[0046] 1.1 Drugs and reagents
[0047] Tested drugs: commercially available Cistanche medicinal materials, Cistanche total glycosides extract I (Example 5), Cistanche total glycosides extract II (Example 6);
[0048] Panax ginseng granule, Jinri Pharmaceutical Co., Ltd.;
[0049] Urea nitrogen test box, Nanjing Jiancheng Bioengineering Research Institute;
[0050] Whole blood lactic acid assay kit, Nanjing Jiancheng Bioengineering Institute.
[0051] 1.2 Animals
[0052] ICR mice, weighing 18-22 g, male, SPF grade, provided by Beijing Weitong Lihua, license number: SCXK (Beijing) 2016-0011.
[0053] 1.3 Instruments
[0054] Morris water maze, Spanish Panlab company.
[0055] 2. Experimental method
[0056] 50 male ICR mice were randomly divided into 5 groups according to their body weight, including 10 blank control groups (administered w...
Embodiment 3
[0069] Embodiment 3: Experimental research on the sedative effect of the invented test substance on mice
[0070] 1. Experimental materials
[0071] 1.1 Drugs and reagents
[0072] Tested drugs: commercially available Cistanche medicinal materials, Cistanche total glycosides extract I (Example 5), Cistanche total glycosides extract II (Example 6);
[0073] Diazepam, Tianjin Lisheng Pharmaceutical Co., Ltd.
[0074] 1.2 Animals
[0075] ICR mice, weighing 18-22 g, male and female, SPF grade, provided by Beijing Weitong Lihua, license number: SCXK (Beijing) 2016-0011.
[0076] 1.3 Instruments
[0077] YLS-1A multi-function mouse autonomic activity recorder, Jinan Yiyan Technology Development Co., Ltd.;
[0078] Stopwatch, Dongsiyi (Beijing) Technology Co., Ltd.
[0079] 2. Experimental method
[0080] Before the experiment, it was first necessary to screen out that there was no significant difference in the number of autonomous activities of the mice in each group, that i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com