Method for qualitative and quantitative detection on glycoside structured surfactant
A quantitative detection method and surfactant technology, applied in the field of qualitative and quantitative detection of glycoside structure surfactants, can solve the problems of no response signal, inability to realize qualitative identification and quantitative detection, difficulty in separating glycoside structure surfactants, etc. , to achieve a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
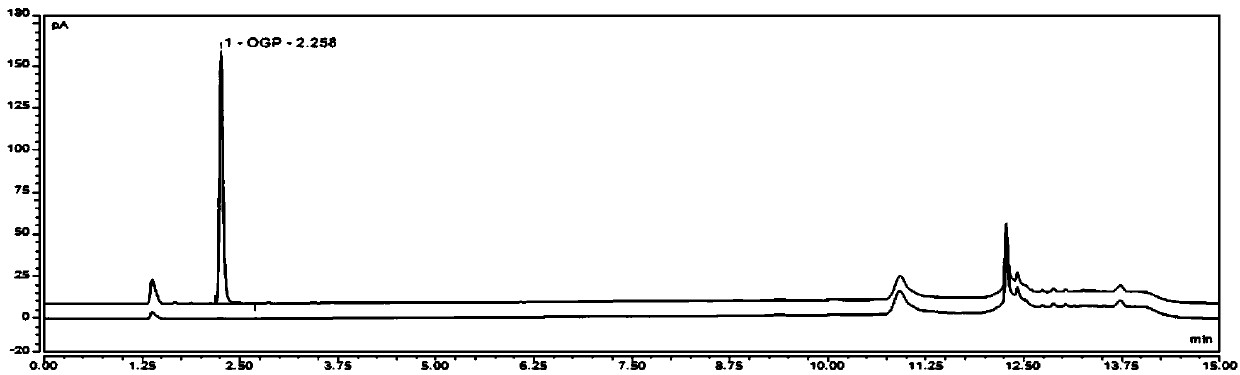
[0047] Embodiment 1 takes the sample of testing octyl-β-D-glucopyranoside (OGP) as an example:
[0048] 1. Mobile phase preparation:
[0049] 1.1. Mobile phase A: 0.1% v / v TFA (trifluoroacetic acid) in 10% v / vacetonitrile (acetonitrile);
[0050] Take 100mL of acetonitrile into a 1000mL solvent bottle, add 900mL of ultrapure water and 1mL of trifluoroacetic acid, and mix well;
[0051] 1.2. Mobile phase B: 0.1% v / v TFAin 90% v / vacetonitrile;
[0052] Take 900mL of acetonitrile into a 1000mL solvent bottle, add 100mL of ultrapure water and 1mL of trifluoroacetic acid, and mix well;
[0053] 1.3. Mobile phase C: pure water
[0054] Take 1L of pure water in a 1L solvent bottle;
[0055] 1.4. Mobile phase D: acetonitrile (column preservation solution);
[0056] Take 1L of acetonitrile in a 1L solvent bottle;
[0057] 1.5.Seal wash (cleaning the plunger rod seal): 10% IPA
[0058] Take 100mL isopropanol (IPA) and 900mL ultrapure water in a 1L solvent bottle and mix thoroughl...
Embodiment 2
[0097] Embodiment 2 takes the sample of testing decyl-β-D-glucopyranoside (DGP) as an example:
[0098] The experimental procedure of embodiment 2 is with embodiment 1, and experimental result is as follows:
[0099] 4. Test results
[0100] The reverse chromatogram of a sample containing decyl-β-D-glucopyranoside is shown in Figure 2-A As shown, decyl-β-D-glucopyranoside (injection volume 50 μL,) can be separated from the solvent peak with a retention time of 3.7 minutes.
[0101] 5. Glycosides are separated by a reverse chromatographic column and then enter a charged aerosol detector for detection
[0102] This embodiment uses a liquid phase-detector combination system, so when the glycosides are separated by the reverse chromatographic column, they will automatically enter the charged aerosol (CAD) detector through the pipeline.
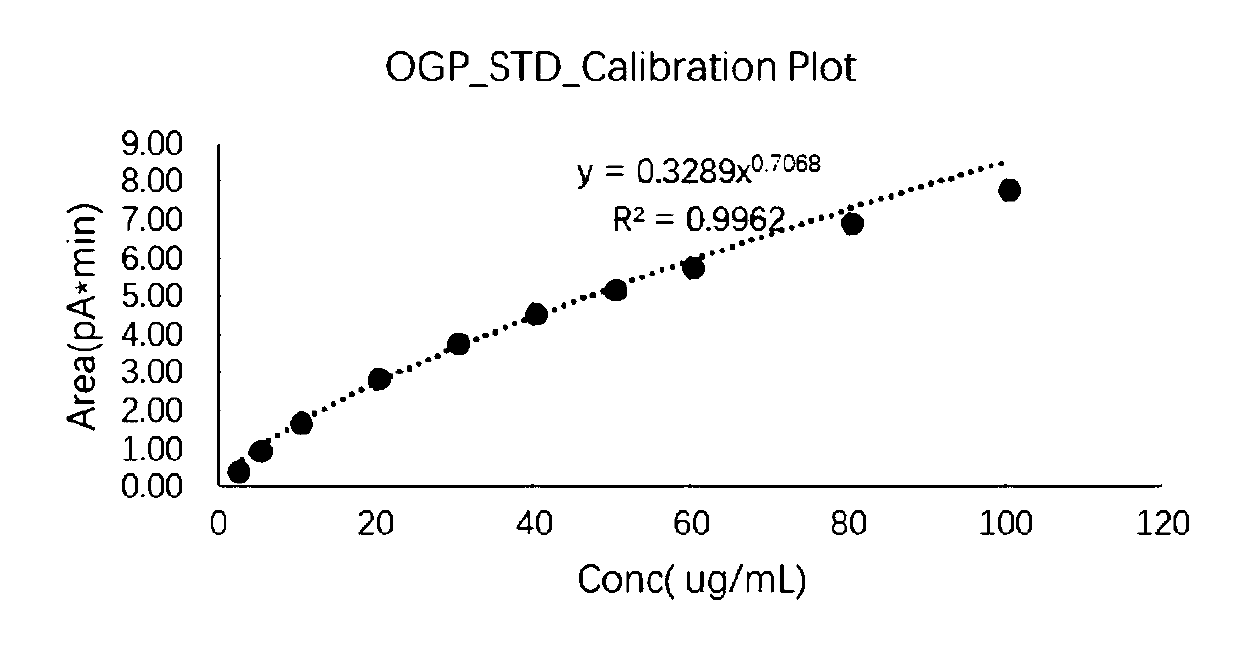
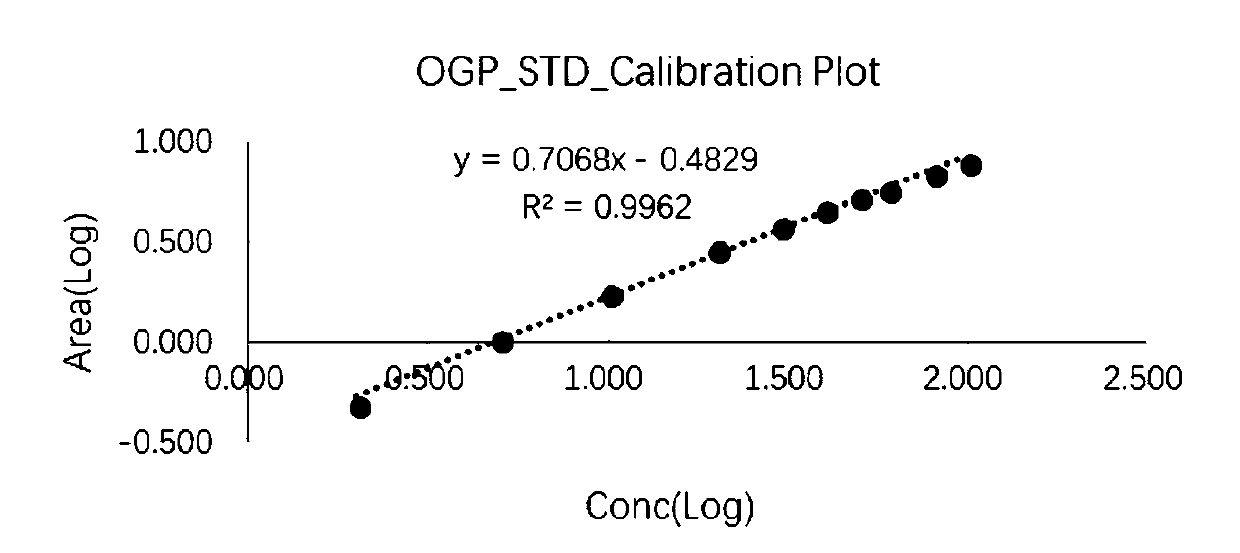
[0103] The signal response was determined for the standard solution of decyl-β-D-glucopyranoside at different concentrations, and the logarit...
Embodiment 3
[0111] Embodiment 3 takes the sample of testing dodecyl-β-D-maltopyranoside (DMP) as an example:
[0112] The experimental procedure of embodiment 3 is with embodiment 1, and experimental result is as follows:
[0113] 4. Test results
[0114] The reverse chromatogram of a sample containing dodecyl-β-D-maltopyranoside is shown in Figure 3-A As shown, dodecyl-β-D-maltopyranoside (injection volume 50 μL,) can be separated from the solvent peak with a retention time of 5.3 minutes.
[0115] 5. Glycosides are separated by a reverse chromatographic column and then enter a charged aerosol detector for detection
[0116] This embodiment uses a liquid phase-detector combination system, so when the glycosides are separated by the reverse chromatographic column, they will automatically enter the charged aerosol (CAD) detector through the pipeline.
[0117] The signal response was measured for the standard solution of dodecyl-β-D-maltopyranoside at different concentrations, and the l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com