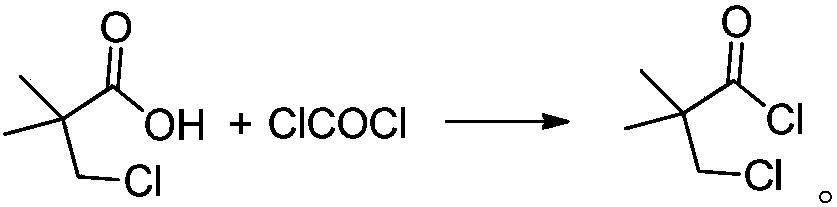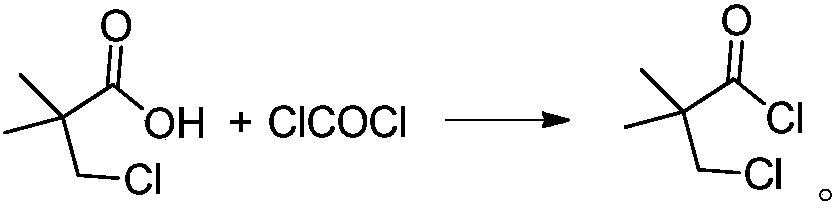Method for preparing 3-chloro-2,2-dimethylpropionyl chloride
A technology of dimethylpropionyl chloride and dimethylpropionic acid, which is applied in the field of preparation of 3-chloro-2,2-dimethylpropionyl chloride, can solve the problems of harsh reaction conditions and long reaction time, and achieve mild conditions. Easy to control, low cost, high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 200g of 3-chloro-2,2-dimethylpropionic acid into the reaction kettle, raise the temperature to 50°C, heat it for 0.5h after melting, add 10g of DMF, continue to keep it for 0.5h, in the range of 50-60°C, feed 150g of phosgene gas, 2.0h ventilation is completed, then maintain the temperature of the kettle at 50-60°C, degas under reduced pressure (vacuum degree of 100mbar) for 2h, and finally obtain 222.93g of 3-chloro-2,2-dimethylpropionyl chloride, the yield 98.2%, gas chromatography detection product purity A% is 98.7%. That 1 H NMR (CDCl 3 ): δ3.61(s, 2H), δ1.39(s, 6H); IR(KBr) main absorption peaks are 2987, 2942, 1811, 1791, 1471, 1391, 923, 880, 811cm -1 ; 1 Both H NMR and IR agree well with the standard spectra.
Embodiment 2
[0034] Add 200g of 3-chloro-2,2-dimethylpropionic acid into the reaction kettle, raise the temperature to 70°C, heat it for 0.5h after melting, add 20g of DMF, continue to keep it for 0.5h, in the range of 70-80°C, feed 148g of phosgene gas, 3.0h ventilation is completed, then maintain the temperature of the kettle at 70-80°C, degas under reduced pressure (vacuum degree 100mbar) for 0.5h, and finally obtain 221.57g of 3-chloro-2,2-dimethylpropionyl chloride, The yield was 97.6%, and the product purity A% detected by gas chromatography was 98.2%. Identification data is with embodiment 1, 1 Both H NMR and IR agree well with the standard spectra.
Embodiment 3
[0036] Add 200g of 3-chloro-2,2-dimethylpropionic acid into the reaction kettle, raise the temperature to 90°C, heat it for 0.5h after melting, add 15g of DMF, keep the heat for 0.5h, in the range of 90-95°C, feed 155g of phosgene gas, 2.0h ventilation is completed, then maintain the temperature of the kettle at 90-95 ° C, degassing under reduced pressure (vacuum degree of 100mbar) for 0.5h, and finally obtain 224.75g of 3-chloro-2,2-dimethylpropionyl chloride, received The yield is 99.0%, and the product purity A% detected by gas chromatography is 98.9%. Identification data is with embodiment 1, 1 Both H NMR and IR agree well with the standard spectra.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com