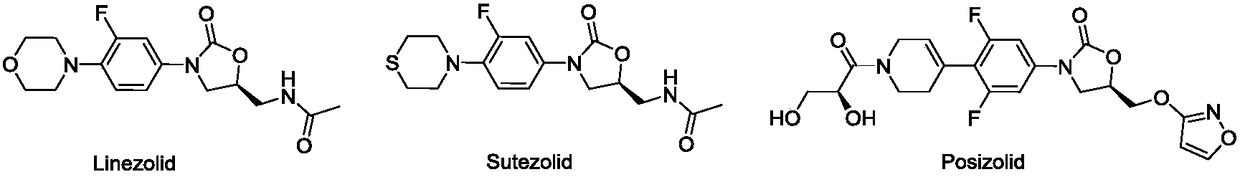
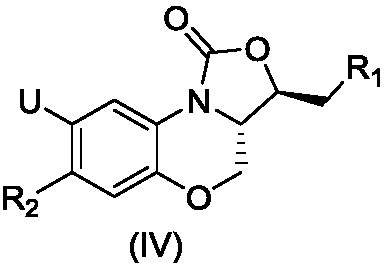
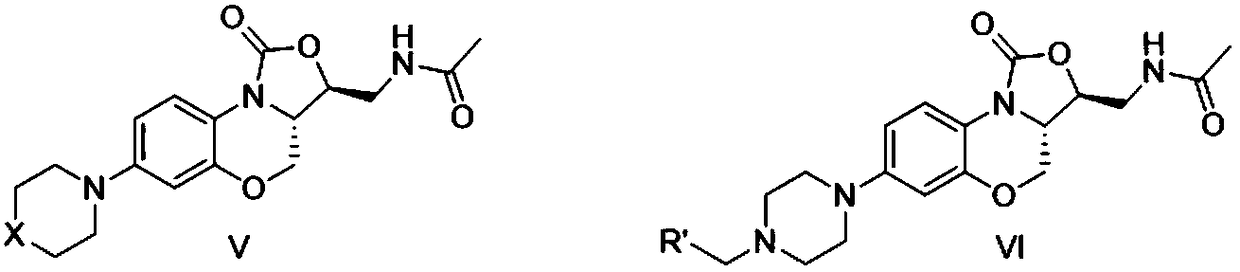
Nitrogen heterocyclic ring substituent containing benzoxazine oxazolidinone compound as well as preparation method and application thereof
A compound, unsubstituted technology for use in medicine to address issues such as safety and efficacy interruption studies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0216] Preparation of ((2R,3S)-3-(((tert-butyldimethylsilyl)oxy)methyl)epoxy-2-yl)methanol (Intermediate 1)
[0217]
[0218] Add 4A molecular sieves (7.2 g) and anhydrous dichloromethane (180 mL) into a 500 mL three-necked flask, protect the system with Ar, and cool down to -20°C. D-(-)-Diethyl tartrate (7.8 mL, 45.7 mmol) was added, followed by dropwise addition of tetraisopropyl titanate (12 mL, 39.8 mmol), and the system turned yellow. After stirring for 0.5 h, (Z)-4-((tert-butyldimethylsilyl)oxy)but-2-en-1-ol (12 g, 59.4 mmol) was added and stirred for 1 h. A toluene solution of tert-butanol peroxide (5M, 28.5 mL, 142 mmol) was added dropwise and kept stirring overnight. After TLC monitors the reaction, add FeSO-containing 4 ·7H 2 A tartaric acid solution (10%, 192 mL) of O (23.4 g, 84 mmol) was stirred at 0° C. for 5 hours, and then the layers were separated. Filter, separate the organic phase, extract the aqueous phase once with dichloromethane, and combine the o...
preparation example 2
[0220] Preparation of ((2S,3R)-3-(((tert-butyldimethylsilyl)oxy)methyl)epoxy-2-yl)methanol (intermediate 2)
[0221]
[0222] In the preparation method of intermediate 1, D-(-)-diethyl tartrate was replaced by L-(+)-diethyl tartrate, and the reaction was carried out to obtain intermediate 2, 3.0 g of light yellow oil, yield 55.6%.
preparation example 3
[0224] Preparation of ((2R,3S)-3-((trityloxy)methyl)epoxy-2-yl)methanol (intermediate 3)
[0225]
[0226] Add 4A molecular sieves (12 g) and anhydrous dichloromethane (330 mL) into a 1 L four-necked flask, protect the system with Ar, and cool down to -40°C. D-(-)-diethyl tartrate (13.6 mL, 79.2 mmol) was added, followed by dropwise addition of tetraisopropyl titanate (18.8 mL, 63.4 mmol), and the system turned yellow. After stirring for 0.5 h, add (Z)-4-(trityloxy)but-2-en-1-ol (26.1 g, 79.2 mmol) in dichloromethane (120 mL), and stir for 0.5 h. A toluene solution of tert-butanol peroxide (3.8M, 50 mL, 190 mmol) was added dropwise and kept stirring for 2 h, and stirred overnight at -20°C. After TLC monitors the reaction, add FeSO-containing 4 ·7H2 A tartaric acid solution (10%, 200 mL) of O (30 g) was stirred at 0°C for 1 hour, and then the layers were separated. The organic phase was separated, and the solution phase was extracted twice with dichloromethane, and the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com