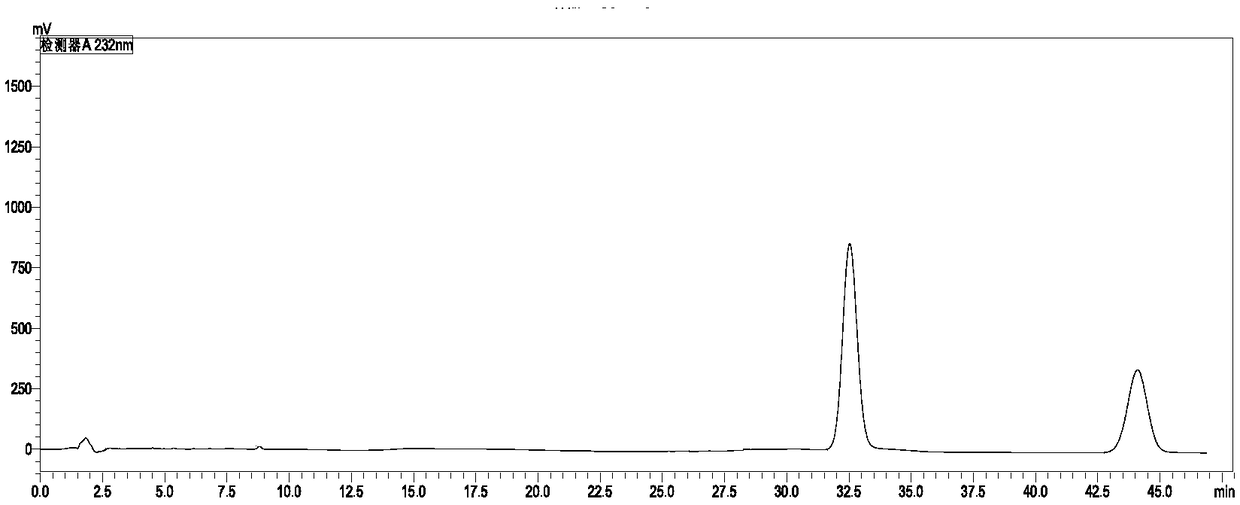
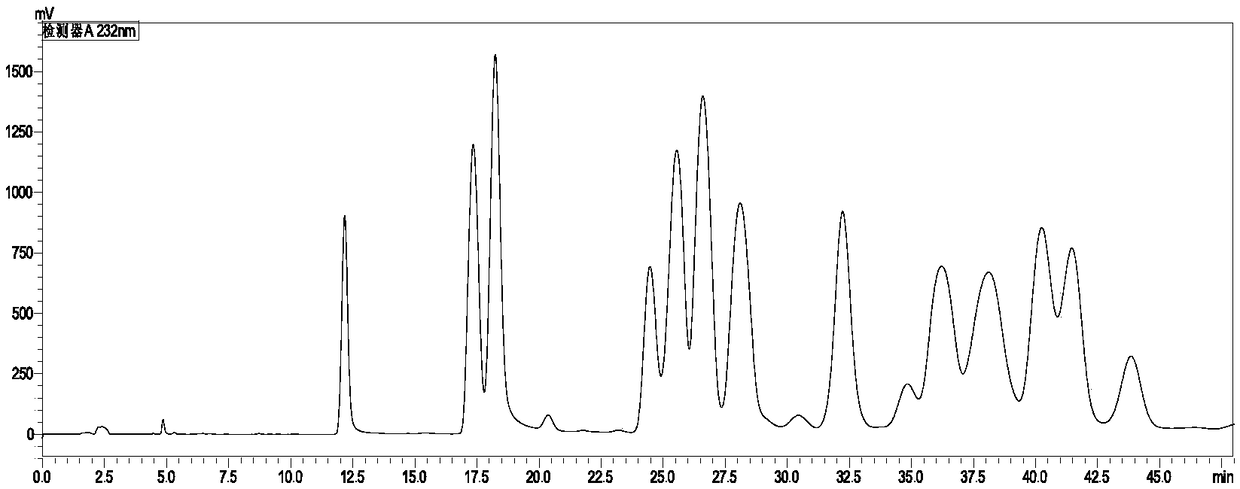
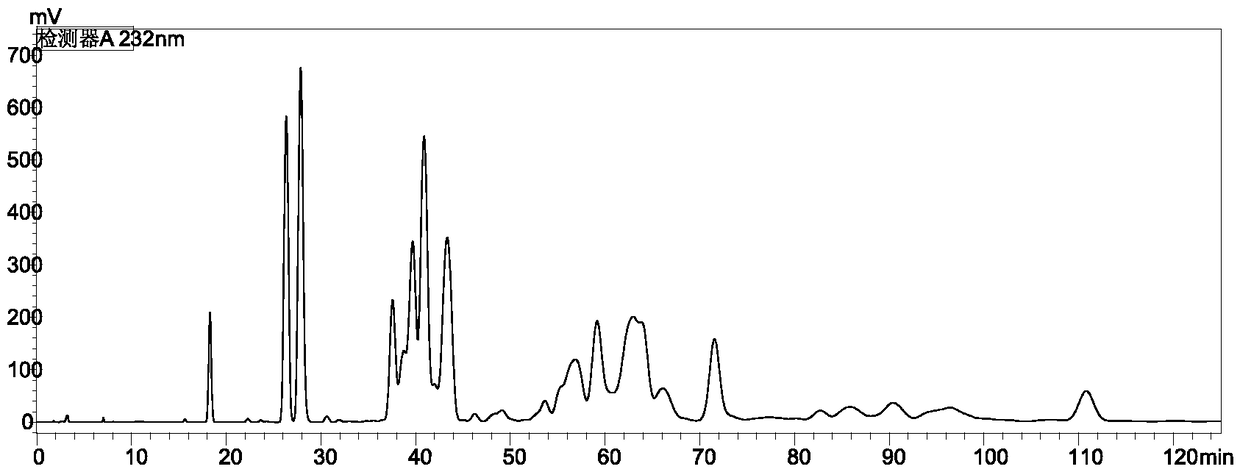
Method for detecting content of cyhalofop-butyl and pyribenzoxim in compound preparation
A technology of pyrimidifen and cyhalofop-butyl, applied in the field of analytical chemistry, can solve the problems of inseparability, time-consuming, waste of reagents and the like, and achieve the effects of high sensitivity, good reproducibility and accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The chromatographic conditions for detection are as follows:
[0038] Variable wavelength ultraviolet detector, C18 chromatographic column 5.0μm250mm×4.6mm (I.D.);
[0039] Mobile phase: acetonitrile: water = 60: 40;
[0040] Detection wavelength: 232nm;
[0041] Flow rate: 1.0mL / min;
[0042] The specific detection method is:
[0043] Accurately weigh 0.05g of the standard cyhalofop-methyl and 0.0210g of saflufenacil in the same 50mL volumetric flask, dissolve and dilute to the mark with acetonitrile. Weigh about 0.8100g of a sample of 60g / L cyhalofop-methyl + 25g / L saflufenacil compound EC, place it in a 50mL volumetric flask, dissolve it with acetonitrile and dilute to the mark, and degas it by ultrasonic for 5 minutes. The standard sample was taken for liquid chromatography analysis, the injection volume was 5 μL, and the peak areas of cyhalofop-ethyl and saflufenacil were determined. Samples were taken for liquid chromatography analysis with an injection volum...
Embodiment 2
[0049]In order to verify the feasibility and accuracy of the detection method of Example 1, a precision test was carried out. The test adopted a concentration of 1000 mg / L of cyhalofop-ester and saflufenacil standard solution prepared, and 5 μL of the standard solution was continuously injected 6 times. The measurement results are shown in Table 2.
[0050] Table 2
[0051] serial number
[0052] It can be drawn from Table 2 that the RSD of the peak area is less than 2%, so this method has good stability.
Embodiment 3
[0054] In order to better illustrate the stability of the sample solution containing cyhalofop-ethyl and saflufenacil provided in the examples of the present invention, the sample solution was prepared and placed for 4h, 8h, 12h, and 24h respectively, and then analyzed by liquid chromatography. The results are shown in Table 3.
[0055] table 3
[0056]
[0057]
[0058] As can be seen from Table 3, the RSD of the peak area is less than 2%, and the stability of the test solution provided by the present invention is good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com