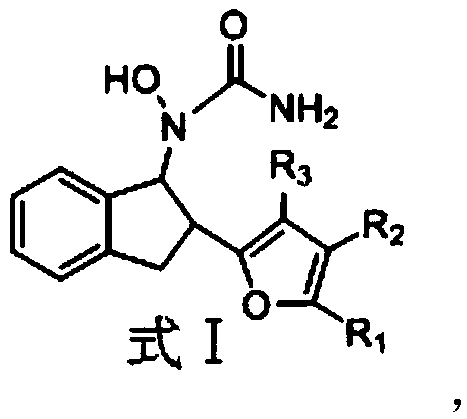
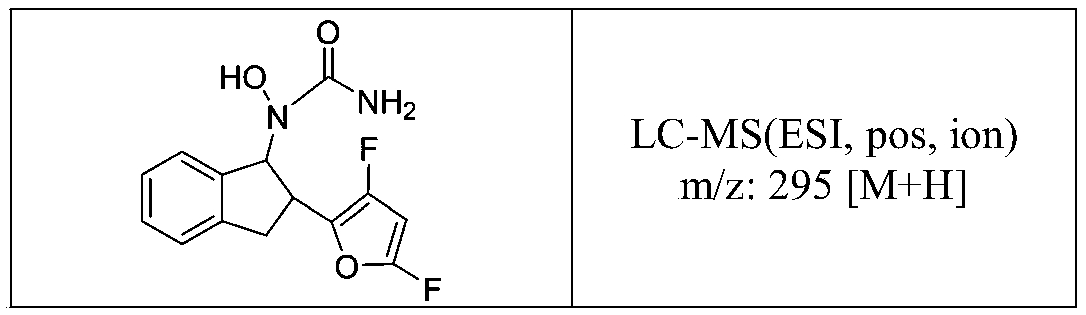
Urea derivative and application thereof to prevention and treatment of inflammation
A technology of derivatives and ureas, applied in the field of urea derivatives and their application in the prevention and treatment of inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of 1-hydroxyl-1-(2-(pyrrol-2-yl)-2,3-dihydro-1H-inden-1-yl)urea
[0028] Synthesis of 1-1,2-bromo-2,3-dihydro-1H-inden-1-ylmethanesulfonic acid:
[0029]
[0030] To a solution of 2-bromo-2,3-dihydro-1H-inden-1-ol (10.0 g, 46.93 mmol), triethylamine (10.8 g, 106.73 mmol) in DCM (50 mL) at 0 °C was added dropwise Methanesulfonyl chloride MsCl (9.9 g, 86.43 mmol) was added dropwise over 30 minutes. The mixture was stirred for an additional 2 hours, then allowed to warm to room temperature. Water (50 mL) was added, the DCM layer was separated and the aqueous phase was extracted twice more with DCM (2 x 30 mL). The combined organic phases were washed with brine (50 mL), then washed with Na 2 SO 4 dry. The solvent was removed under vacuum to give 2-bromo-2,3-dihydro-1H-inden-1-ylmethanesulfonic acid as a light oil, yield 13.53 g, 99% yield, which was directly obtained without further purification for the next step. 1 H-NMR (400MHz, CDCl3) δ: 3....
Embodiment 2
[0040] Example 2: Synthesis of 1-hydroxyl-1-(2-(5-fluoro-pyrrol-2-yl)-2,3-dihydro-1H-inden-1-yl)urea:
[0041]
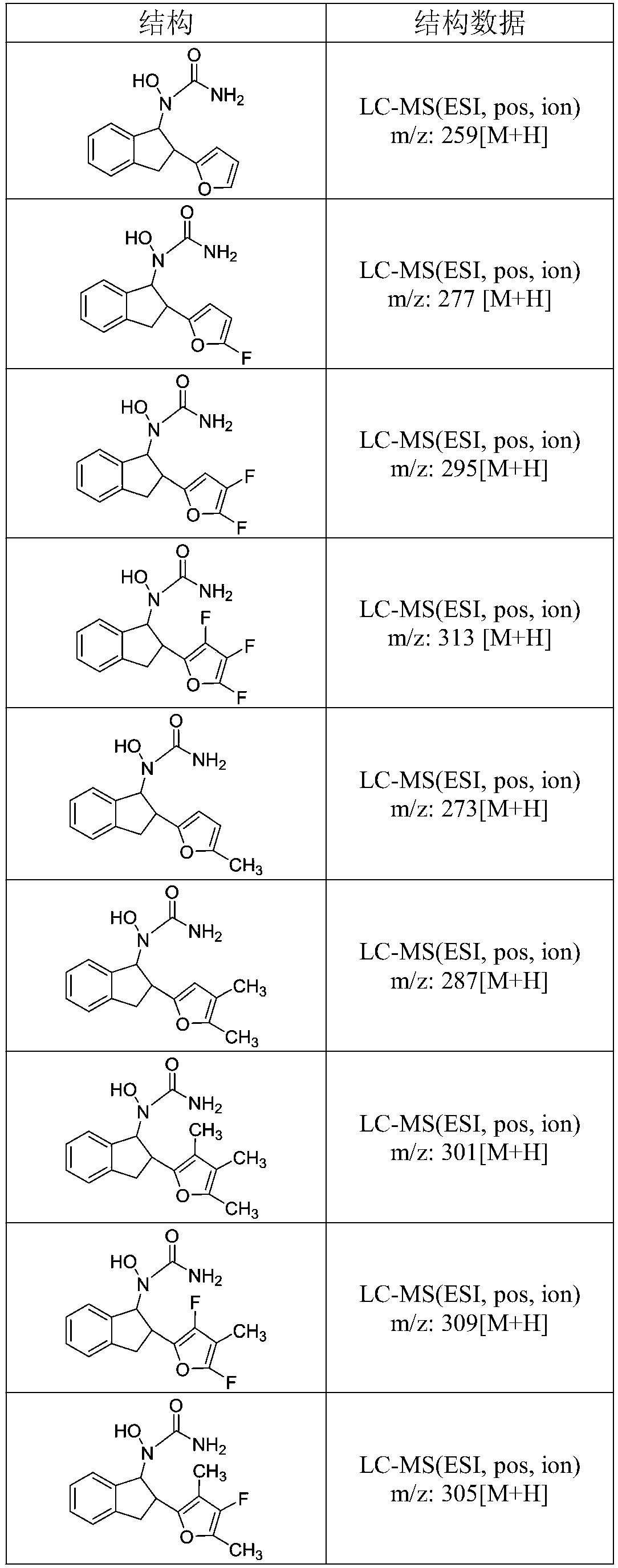
[0042] 1-(2-Bromo-2,3-dihydro-1H-inden-1-yl)-1-hydroxyurea (10.77 g, 39.73 mmol) was dissolved in 96 mL of H 2 O:EtOH (1:1) mixture and placed in a three-necked flask (250mL). 5-Fluoro-pyrrole-2-boronic acid (55.47 mmol) and potassium carbonate (8.29 g, 59.99 mmol) were added to the mixture. Then PdNPs catalyst (0.4 mmol% Pd) was added, and the mixture was vigorously stirred at 60 °C for 10 min under nitrogen atmosphere. The reaction mixture was added to 0.2 mol / L sodium hydroxide solution (55 mL) and extracted with ethyl acetate (40 mL). The organic layers were combined and crystallized by volatilization in air to obtain the solid product 1-hydroxyl-1-(2-(5-fluoro-pyrrol-2-yl)-2,3-dihydro-1H-inden-1-yl) Urea, 9.32 g, 85% yield. LC-MS (ESI, pos, ion) m / z: 277 [M+H].
Embodiment 3
[0043] Example 3: Synthesis of 1-hydroxyl-1-(2-(5-methyl-pyrrol-2-yl)-2,3-dihydro-1H-inden-1-yl)urea:
[0044]
[0045] 1-(2-Bromo-2,3-dihydro-1H-inden-1-yl)-1-hydroxyurea (10.77 g, 39.73 mmol) was dissolved in 96 mL of H 2 O:EtOH (1:1) mixture and placed in a three-necked flask (250mL). 5-Methyl-pyrrole-2-boronic acid (55.47 mmol) and potassium carbonate (8.29 g, 59.99 mmol) were added to the mixture. Then PdNPs catalyst (0.4 mmol% Pd) was added, and the mixture was vigorously stirred at 60 °C for 10 min under nitrogen atmosphere. The reaction mixture was added to 0.2 mol / L sodium hydroxide solution (55 mL) and extracted with ethyl acetate (40 mL). The organic layers were combined and evaporated and crystallized in the air to obtain the solid product 1-hydroxyl-1-(2-(5-methyl-pyrrol-2-yl)-2,3-dihydro-1H-inden-1-yl ) urea, 8.75g, the yield was 81%. LC-MS (ESI, pos, ion) m / z: 273 [M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com