Synthesis method of cefalexin impurity
A synthesis method, the technology of cephalexin, is applied in the direction of organic chemistry, etc., to achieve the effects of high content of synthetic products, reduction of the generation of by-products, and simple operation of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
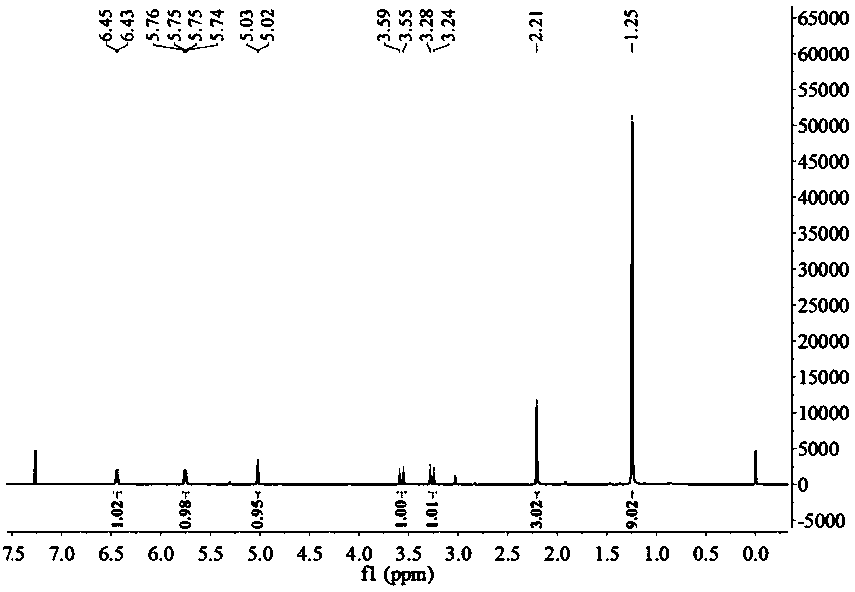
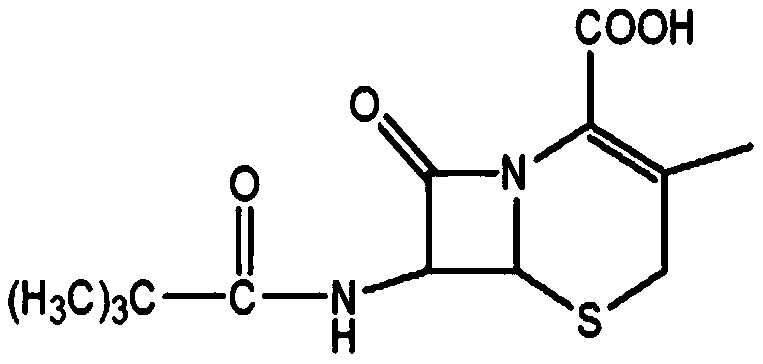
[0029] This example relates to the synthesis method of cephalexin impurity, the cephalexin impurity is specifically (6R,7R)-7-[(2,2-dimethyl-1-oxopropyl)amino]-3-methyl- 8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, and the synthetic method comprises:
[0030] Step a, at 0-10°C, react 7-aminodesacetoxycephalosporanic acid with a molar ratio of 1:(1-1.5) and tetramethylguanidine for 0.5-1 hour to obtain 7-aminodesacetoxy Cephalosporanate.
[0031] Step b, at 0-25°C, react 7-aminodesacetoxycephalosporanate with a molar ratio of 1:(0.5-1.5) and pivaloyl chloride for 2-5 hours to obtain (6R,7R)- 7-[(2,2-Dimethyl-1-oxopropyl)amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]octane-2 -ene-2-carboxylates.
[0032] Step c, at 15-40°C, fully mix dilute hydrochloric acid with a mass concentration of 1%-15% and the product obtained in step b, carry out a hydrolysis reaction at 25-35°C for 10-60 minutes, and statically Set the layers, remove the lower organic phase and ev...
preparation example 1
[0038] The synthesis of the cephalexin impurity of present example comprises the following steps.
[0039] Step a, in a dry and clean reaction flask, add 40ml of dichloromethane and 5g of 7-aminodesacetoxy cephalosporanic acid, cool to 0°C, add 2.8g of tetramethylguanidine in the reaction flask, control the reaction temperature Above 10°C, react for 30 minutes to obtain 7-aminodesacetoxycephalosporanic acid salt solution.
[0040] Step b. Add 3 g of pivaloyl chloride to the reaction flask under temperature control not exceeding 10° C. After the addition, control the temperature at 0° C. and react for 4 hours.
[0041] Step c, add 70ml of hydrochloric acid with a mass concentration of 5.64% to the hydrolysis reaction bottle, adjust the temperature to 30°C, pour the product after the reaction in step b into the hydrolysis reaction bottle and stir for 30 minutes, let it stand for 30 minutes, and remove the lower layer Organic phase, the organic phase was evaporated and crystalli...
preparation example 2
[0044] The synthesis of the cephalexin impurity of present example comprises the following steps.
[0045] Step a, in a dry and clean reaction flask, add 10ml of dichloromethane, 30ml of chloroform and 5g of 7-aminodesacetoxycephalosporanic acid, cool to 4°C, add 3.2g of tetramethylguanidine into the reaction flask, Control the reaction temperature not to be higher than 10° C., and react for 40 minutes to obtain a 7-aminodesacetoxycephalosporanic acid salt solution.
[0046] Step b. Add 2 g of pivaloyl chloride to the reaction flask under temperature control not exceeding 10° C. After the addition, control the temperature at 4° C. and react for 3 hours.
[0047] Step c, add 70ml of hydrochloric acid with a mass concentration of 2.84% to the hydrolysis reaction bottle, adjust the temperature to 35°C, pour the product after the reaction in step b into the hydrolysis reaction bottle and stir for 40 minutes, let it stand for 30 minutes, and remove the lower layer Organic phase, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com